WWP1 E3 ligase at the crossroads of health and disease
- PMID: 38129384
- PMCID: PMC10739765
- DOI: 10.1038/s41419-023-06380-0
WWP1 E3 ligase at the crossroads of health and disease
Abstract
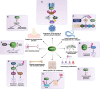
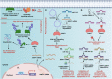
The E3 ubiquitin ligase WWP1 (WW Domain-containing E3 Ubiquitin Protein Ligase 1) is a member of the HECT (Homologous to the E6-associated protein Carboxyl Terminus) E3 ligase family. It is conserved across several species and plays crucial roles in various physiological processes, including development, cell growth and proliferation, apoptosis, and differentiation. It exerts its functions through ubiquitination or protein-protein interaction with PPXY-containing proteins. WWP1 plays a role in several human diseases, including cardiac conditions, neurodevelopmental, age-associated osteogenic disorders, infectious diseases, and cancers. In solid tumors, WWP1 plays a dual role as both an oncogene and a tumor suppressor, whereas in hematological malignancies such as AML, it is identified as a dedicated oncogene. Importantly, WWP1 inhibition using small molecule inhibitors such as Indole-3-Carbinol (I3C) and Bortezomib or siRNAs leads to significant suppression of cancer growth and healing of bone fractures, suggesting that WWP1 might serve as a potential therapeutic _target for several diseases. In this review, we discuss the evolutionary perspective, structure, and functions of WWP1 and its multilevel regulation by various regulators. We also examine its emerging roles in cancer progression and its therapeutic potential. Finally, we highlight WWP1's role in normal physiology, contribution to pathological conditions, and therapeutic potential for cancer and other diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Ubiquitin Ligase WWP1 Interacts with Ebola Virus VP40 To Regulate Egress.J Virol. 2017 Sep 27;91(20):e00812-17. doi: 10.1128/JVI.00812-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768865 Free PMC article.
-
CYYR1 promotes the degradation of the E3 ubiquitin ligase WWP1 and is associated with favorable prognosis in breast cancer.J Biol Chem. 2024 Sep;300(9):107601. doi: 10.1016/j.jbc.2024.107601. Epub 2024 Jul 24. J Biol Chem. 2024. PMID: 39059493 Free PMC article.
-
WWP1 E3 ligase _targets LATS1 for ubiquitin-mediated degradation in breast cancer cells.PLoS One. 2013;8(4):e61027. doi: 10.1371/journal.pone.0061027. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23573293 Free PMC article.
-
WW Domain-Containing E3 Ubiquitin Protein Ligase 1: A Self-Disciplined Oncoprotein.Front Cell Dev Biol. 2021 Oct 12;9:757493. doi: 10.3389/fcell.2021.757493. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34712671 Free PMC article. Review.
-
WWP1: a versatile ubiquitin E3 ligase in signaling and diseases.Cell Mol Life Sci. 2012 May;69(9):1425-34. doi: 10.1007/s00018-011-0871-7. Epub 2011 Nov 4. Cell Mol Life Sci. 2012. PMID: 22051607 Free PMC article. Review.
Cited by
-
Indole-3-Carbinol and Its Derivatives as Neuroprotective Modulators.Brain Sci. 2024 Jul 2;14(7):674. doi: 10.3390/brainsci14070674. Brain Sci. 2024. PMID: 39061415 Free PMC article. Review.
-
The R436Q missense mutation in WWP1 disrupts autoinhibition of its E3 ubiquitin ligase activity, leading to self-degradation and loss of function.In Vitro Cell Dev Biol Anim. 2024 Aug;60(7):771-780. doi: 10.1007/s11626-024-00894-3. Epub 2024 Apr 1. In Vitro Cell Dev Biol Anim. 2024. PMID: 38561589
-
Immunohistochemical Expression and Clinical Significance of WWP1 Protein in Nasopharyngeal Cancer.J Histochem Cytochem. 2024 Jun;72(6):363-371. doi: 10.1369/00221554241255722. Epub 2024 May 28. J Histochem Cytochem. 2024. PMID: 38804681
-
PTMs of PD-1/PD-L1 and PROTACs application for improving cancer immunotherapy.Front Immunol. 2024 Apr 4;15:1392546. doi: 10.3389/fimmu.2024.1392546. eCollection 2024. Front Immunol. 2024. PMID: 38638430 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

