Protease-Sensitive and -Resistant Forms of Human and Murine Alpha-Synucleins in Distinct Brain Regions of Transgenic Mice (M83) Expressing the Human Mutated A53T Protein
- PMID: 38136658
- PMCID: PMC10741842
- DOI: 10.3390/biom13121788
Protease-Sensitive and -Resistant Forms of Human and Murine Alpha-Synucleins in Distinct Brain Regions of Transgenic Mice (M83) Expressing the Human Mutated A53T Protein
Abstract
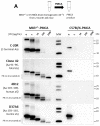
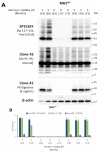
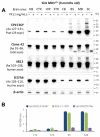
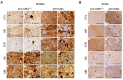
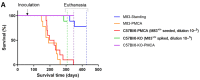
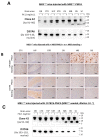
Human neurodegenerative diseases associated with the misfolding of the alpha-synuclein (aS) protein (synucleinopathies) are similar to prion diseases to the extent that lesions are spread by similar molecular mechanisms. In a transgenic mouse model (M83) overexpressing a mutated (A53T) form of human aS, we had previously found that Protein Misfolding Cyclic Amplification (PMCA) triggered the aggregation of aS, which is associated with a high resistance to the proteinase K (PK) digestion of both human and murine aS, a major hallmark of the disease-associated prion protein. In addition, PMCA was also able to trigger the aggregation of murine aS in C57Bl/6 mouse brains after seeding with sick M83 mouse brains. Here, we show that intracerebral inoculations of M83 mice with C57Bl/6-PMCA samples strikingly shortens the incubation period before the typical paralysis that develops in this transgenic model, demonstrating the pathogenicity of PMCA-aggregated murine aS. In the hind brain regions of these sick M83 mice containing lesions with an accumulation of aS phosphorylated at serine 129, aS also showed a high PK resistance in the N-terminal part of the protein. In contrast to M83 mice, old APPxM83 mice co-expressing human mutated amyloid precursor and presenilin 1 proteins were seen to have an aggregation of aS, especially in the cerebral cortex, hippocampus and striatum, which also contained the highest load of aS phosphorylated at serine 129. This was proven by three techniques: a Western blot analysis of PK-resistant aS; an ELISA detection of aS aggregates; or the identification of aggregates of aS using immunohistochemical analyses of cytoplasmic/neuritic aS deposits. The results obtained with the D37A6 antibody suggest a higher involvement of murine aS in APPxM83 mice than in M83 mice. Our study used novel tools for the molecular study of synucleinopathies, which highlight similarities with the molecular mechanisms involved in prion diseases.
Keywords: Lewy Body Disease; PMCA; Parkinson’s disease; α-synuclein; β-amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Seeded propagation of α-synuclein aggregation in mouse brain using protein misfolding cyclic amplification.FASEB J. 2019 Nov;33(11):12073-12086. doi: 10.1096/fj.201900354R. Epub 2019 Aug 1. FASEB J. 2019. PMID: 31370680 Free PMC article.
-
Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological α-synuclein by enhanced ELISA.Acta Neuropathol Commun. 2014 Mar 13;2:29. doi: 10.1186/2051-5960-2-29. Acta Neuropathol Commun. 2014. PMID: 24624994 Free PMC article.
-
α-Synuclein strain propagation is independent of cellular prion protein expression in a transgenic synucleinopathy mouse model.PLoS Pathog. 2024 Sep 12;20(9):e1012517. doi: 10.1371/journal.ppat.1012517. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39264912 Free PMC article.
-
Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
-
Neuropathology and molecular diagnosis of Synucleinopathies.Mol Neurodegener. 2021 Dec 18;16(1):83. doi: 10.1186/s13024-021-00501-z. Mol Neurodegener. 2021. PMID: 34922583 Free PMC article. Review.
References
-
- Irwin D.J., Grossman M., Weintraub D., Hurtig H.I., Duda J.E., Xie S.X., Lee E.B., Van Deerlin V.M., Lopez O.L., Kofler J.K., et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017;16:55–65. doi: 10.1016/S1474-4422(16)30291-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

