Transcriptomic profiling of early synucleinopathy in rats induced with preformed fibrils
- PMID: 38172128
- PMCID: PMC10764951
- DOI: 10.1038/s41531-023-00620-y
Transcriptomic profiling of early synucleinopathy in rats induced with preformed fibrils
Abstract
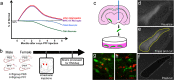
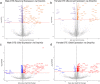
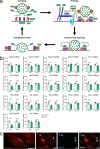
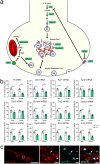
Examination of early phases of synucleinopathy when inclusions are present, but long before neurodegeneration occurs, is critical to both understanding disease progression and the development of disease modifying therapies. The rat alpha-synuclein (α-syn) preformed fibril (PFF) model induces synchronized synucleinopathy that recapitulates the pathological features of Parkinson's disease (PD) and can be used to study synucleinopathy progression. In this model, phosphorylated α-syn (pSyn) inclusion-containing neurons and reactive microglia (major histocompatibility complex-II immunoreactive) peak in the substantia nigra pars compacta (SNpc) months before appreciable neurodegeneration. However, it remains unclear which specific genes are driving these phenotypic changes. To identify transcriptional changes associated with early synucleinopathy, we used laser capture microdissection of the SNpc paired with RNA sequencing (RNASeq). Precision collection of the SNpc allowed for the assessment of differential transcript expression in the nigral dopamine neurons and proximal glia. Transcripts upregulated in early synucleinopathy were mainly associated with an immune response, whereas transcripts downregulated were associated with neurotransmission and the dopamine pathway. A subset of 29 transcripts associated with neurotransmission/vesicular release and the dopamine pathway were verified in a separate cohort of males and females to confirm reproducibility. Within this subset, fluorescent in situ hybridization (FISH) was used to localize decreases in the Syt1 and Slc6a3 transcripts to pSyn inclusion-containing neurons. Identification of transcriptional changes in early synucleinopathy provides insight into the molecular mechanisms driving neurodegeneration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neuroinflammatory gene expression profiles of reactive glia in the substantia nigra suggest a multidimensional immune response to alpha synuclein inclusions.Neurobiol Dis. 2024 Feb;191:106411. doi: 10.1016/j.nbd.2024.106411. Epub 2024 Jan 14. Neurobiol Dis. 2024. PMID: 38228253 Free PMC article.
-
Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration.J Neuroinflammation. 2018 May 1;15(1):129. doi: 10.1186/s12974-018-1171-z. J Neuroinflammation. 2018. PMID: 29716614 Free PMC article.
-
Alpha-synuclein inclusion responsive microglia are resistant to CSF1R inhibition.J Neuroinflammation. 2024 Apr 25;21(1):108. doi: 10.1186/s12974-024-03108-5. J Neuroinflammation. 2024. PMID: 38664840 Free PMC article.
-
A Combined α-Synuclein/Fibril (SynFib) Model of Parkinson-Like Synucleinopathy _targeting the Nigrostriatal Dopamine System.J Parkinsons Dis. 2022;12(8):2307-2320. doi: 10.3233/JPD-223452. J Parkinsons Dis. 2022. PMID: 36189605 Free PMC article. Review.
-
Synucleinopathy-associated pathogenesis in Parkinson's disease and the potential for brain-derived neurotrophic factor.NPJ Parkinsons Dis. 2021 Apr 12;7(1):35. doi: 10.1038/s41531-021-00179-6. NPJ Parkinsons Dis. 2021. PMID: 33846345 Free PMC article. Review.
Cited by
-
Neuroinflammatory gene expression profiles of reactive glia in the substantia nigra suggest a multidimensional immune response to alpha synuclein inclusions.Neurobiol Dis. 2024 Feb;191:106411. doi: 10.1016/j.nbd.2024.106411. Epub 2024 Jan 14. Neurobiol Dis. 2024. PMID: 38228253 Free PMC article.
-
Network analysis of α-synuclein pathology progression reveals p21-activated kinases as regulators of vulnerability.bioRxiv [Preprint]. 2024 Oct 22:2024.10.22.619411. doi: 10.1101/2024.10.22.619411. bioRxiv. 2024. PMID: 39484617 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

