Immunoglobulin replacement products protect against SARS-CoV-2 infection in vivo despite poor neutralizing activity
- PMID: 38175703
- PMCID: PMC10967375
- DOI: 10.1172/jci.insight.176359
Immunoglobulin replacement products protect against SARS-CoV-2 infection in vivo despite poor neutralizing activity
Abstract
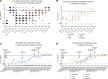
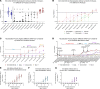
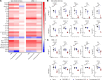
Immunoglobulin (IG) replacement products are used routinely in patients with immune deficiency and other immune dysregulation disorders who have poor responses to vaccination and require passive immunity conferred by commercial antibody products. The binding, neutralizing, and protective activity of intravenously administered IG against SARS-CoV-2 emerging variants remains unknown. Here, we tested 198 different IG products manufactured from December 2019 to August 2022. We show that prepandemic IG had no appreciable cross-reactivity or neutralizing activity against SARS-CoV-2. Anti-spike antibody titers and neutralizing activity against SARS-CoV-2 WA1/2020 D614G increased gradually after the pandemic started and reached levels comparable to vaccinated healthy donors 18 months after the diagnosis of the first COVID-19 case in the United States in January 2020. The average time between production to infusion of IG products was 8 months, which resulted in poor neutralization of the variant strain circulating at the time of infusion. Despite limited neutralizing activity, IG prophylaxis with clinically relevant dosing protected susceptible K18-hACE2-transgenic mice against clinical disease, lung infection, and lung inflammation caused by the XBB.1.5 Omicron variant. Moreover, following IG prophylaxis, levels of XBB.1.5 infection in the lung were higher in FcγR-KO mice than in WT mice. Thus, IG replacement products with poor neutralizing activity against evolving SARS-CoV-2 variants likely confer protection to patients with immune deficiency disorders through Fc effector function mechanisms.
Keywords: COVID-19; Immunoglobulins; Immunology.
Figures


Similar articles
-
SARS-CoV-2 BA.4/5 infection triggers more cross-reactive FcγRIIIa signaling and neutralization than BA.1, in the context of hybrid immunity.J Virol. 2024 Jul 23;98(7):e0067824. doi: 10.1128/jvi.00678-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953380 Free PMC article.
-
Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study.Lancet Infect Dis. 2022 May;22(5):636-648. doi: 10.1016/S1473-3099(21)00764-7. Epub 2022 Jan 25. Lancet Infect Dis. 2022. PMID: 35090638 Free PMC article. Clinical Trial.
-
Exposure of progressive immune dysfunction by SARS-CoV-2 mRNA vaccination in patients with chronic lymphocytic leukemia: A prospective cohort study.PLoS Med. 2023 Jun 29;20(6):e1004157. doi: 10.1371/journal.pmed.1004157. eCollection 2023 Jun. PLoS Med. 2023. PMID: 37384638 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Topical fluoride as a cause of dental fluorosis in children.Cochrane Database Syst Rev. 2024 Jun 20;6(6):CD007693. doi: 10.1002/14651858.CD007693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38899538 Review.
References
-
- Grifols. Plasma journey. https://www.grifols.com/en/plasma-journey Accessed January 11, 2024.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

