Sphingosine 1-Phosphate Regulates Obesity and Glucose Homeostasis
- PMID: 38256005
- PMCID: PMC10816022
- DOI: 10.3390/ijms25020932
Sphingosine 1-Phosphate Regulates Obesity and Glucose Homeostasis
Abstract
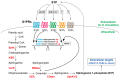
One of the major global health and welfare issues is the treatment of obesity and associated metabolic disorders, such as type 2 diabetes mellitus and nonalcoholic fatty liver disease. Obesity, caused by the excessive accumulation of triglycerides in adipose tissues, induces adipocyte dysfunction, followed by inflammation, in adipose tissues and lipotoxicity in nonadipose tissues. Several studies have shown that obesity and glucose homeostasis are influenced by sphingolipid mediators, including ceramide and sphingosine 1-phosphate (S1P). Cellular accumulation of ceramide impairs pancreatic β-cell survival, confers insulin resistance in the liver and the skeletal muscle, and deteriorates adipose tissue inflammation via unknown molecular mechanisms. The roles of S1P are more complicated, because there are five cell-surface S1P receptors (S1PRs: S1P1-5) which have altered functions, different cellular expression patterns, and inapparent intracellular _targets. Recent findings, including those by our group, support the notable concept that the pharmacological activation of S1P1 or S1P3 improves obesity and associated metabolic disorders, whereas that of S1P2 has the opposite effect. In addition, the regulation of S1P production by sphingosine kinase (SphK) is an essential factor affecting glucose homeostasis. This review summarizes the current knowledge on SphK/S1P/S1PR signaling in and against obesity, insulin resistance, and associated disorders.
Keywords: S1P receptor; adipocyte; adipogenesis; ceramide; glucose tolerance; inflammation; insulin resistance; lipotoxicity; sphingosine 1-phospahte; sphingosine kinase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Ceramide and Sphingosine 1-Phosphate in Liver Diseases.Mol Cells. 2020 May 31;43(5):419-430. doi: 10.14348/molcells.2020.0054. Mol Cells. 2020. PMID: 32392908 Free PMC article. Review.
-
Ceramides and Sphingosino-1-Phosphate in Obesity.Front Endocrinol (Lausanne). 2021 May 13;12:635995. doi: 10.3389/fendo.2021.635995. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34054722 Free PMC article. Review.
-
Sphingosine-1-Phosphate Metabolism in the Regulation of Obesity/Type 2 Diabetes.Cells. 2020 Jul 13;9(7):1682. doi: 10.3390/cells9071682. Cells. 2020. PMID: 32668665 Free PMC article. Review.
-
Sphingosine 1-phosphate metabolism and insulin signaling.Cell Signal. 2021 Jun;82:109959. doi: 10.1016/j.cellsig.2021.109959. Epub 2021 Feb 22. Cell Signal. 2021. PMID: 33631318 Review.
-
Gastric Bypass Surgery Improves the Skeletal Muscle Ceramide/S1P Ratio and Upregulates the AMPK/ SIRT1/ PGC-1α Pathway in Zucker Diabetic Fatty Rats.Obes Surg. 2019 Jul;29(7):2158-2165. doi: 10.1007/s11695-019-03800-z. Obes Surg. 2019. PMID: 30809769
Cited by
-
Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways.Biology (Basel). 2024 Jul 12;13(7):519. doi: 10.3390/biology13070519. Biology (Basel). 2024. PMID: 39056712 Free PMC article. Review.
References
-
- Kitada Y., Kajita K., Taguchi K., Mori I., Yamauchi M., Ikeda T., Kawashima M., Asano M., Kajita T., Ishizuka T., et al. Blockade of Sphingosine 1-Phosphate Receptor 2 Signaling Attenuates High-Fat Diet-Induced Adipocyte Hypertrophy and Systemic Glucose Intolerance in Mice. Endocrinology. 2016;157:1839–1851. doi: 10.1210/en.2015-1768. - DOI - PMC - PubMed
-
- Hong C.H., Ko M.S., Kim J.H., Cho H., Lee C.H., Yoon J.E., Yun J.Y., Baek I.J., Jang J.E., Lee S.E., et al. Sphingosine 1-phosphate receptor 4 promotes nonalcoholic steatohepatitis by activating NLRP3 inflammasome. Cell. Mol. Gastroenterol. Hepatol. 2022;13:925–947. doi: 10.1016/j.jcmgh.2021.12.002. - DOI - PMC - PubMed
-
- Liao C.Y., Barrow F., Venkatesan N., Nakao Y., Mauer A.S., Fredrickson G., Song M.J., Sehrawat T.S., Dasgupta D., Graham R.P., et al. Modulating sphingosine 1-phosphate receptor signaling skews intrahepatic leukocytes and attenuates murine nonalcoholic steatohepatitis. Front. Immunol. 2023;14:1130184. doi: 10.3389/fimmu.2023.1130184. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

