Antidiabetic features of AdipoAI, a novel AdipoR agonist
- PMID: 38269524
- PMCID: PMC10811407
- DOI: 10.1002/cbf.3910
Antidiabetic features of AdipoAI, a novel AdipoR agonist
Abstract
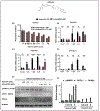
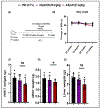
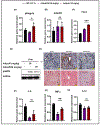
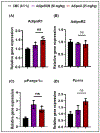
Adiponectin is an antidiabetic endogenous adipokine that plays a protective role against the unfavorable metabolic sequelae of obesity. Recent evidence suggests a sinister link between hypoadiponectinemia and development of insulin resistance/type 2 diabetes (T2D). Adiponectin's insulin-sensitizing property is mediated through the specific adiponectin receptors R1 and R2, which activate the AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR) α pathways. AdipoAI is a novel synthetic analogue of endogenous adiponectin with possibly similar pharmacological effects. Thus, there is a need of orally active small molecules that activate Adipoq subunits, and their downstream signaling, which could ameliorate obesity related type 2 diabetes. In the study we aim to investigate the effects of AdipoAI on obesity and T2D. Through in-vitro and in-vivo analyses, we investigated the antidiabetic potentials of AdipoAI and compared it with AdipoRON, another orally active adiponectin receptors agonist. Our results showed that in-vitro treatment of AdipoAI (0-5 µM) increased adiponectin receptor subunits AdipoR1/R2 with increase in AMPK and APPL1 protein expression in C2C12 myotubes. Similarly, in-vivo, oral administration of AdipoAI (25 mg/kg) observed similar effects as that of AdipoRON (50 mg/kg) with improved control of blood glucose and insulin sensitivity in diet-induced obesity (DIO) mice models. Further, AdipoAI significantly reduced epididymal fat content with decrease in inflammatory markers and increase in PPAR-α and AMPK levels and exhibited hepatoprotective effects in liver. Further, AdipoAI and AdipoRON also observed similar results in adipose tissue. Thus, our results suggest that low doses of orally active small molecule agonist of adiponectin AdipoAI can be a promising therapeutic _target for obesity and T2D.
Keywords: AdipoAI; adiponectin; diet induced obesity; inflammation; obesity; type 2 diabetes.
© 2024 John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
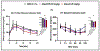
Similar articles
-
A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity.Nature. 2013 Nov 28;503(7477):493-9. doi: 10.1038/nature12656. Epub 2013 Oct 30. Nature. 2013. PMID: 24172895
-
A novel adiponectin receptor agonist (AdipoAI) ameliorates type 2 diabetes-associated periodontitis by enhancing autophagy in osteoclasts.J Periodontal Res. 2022 Apr;57(2):381-391. doi: 10.1111/jre.12969. Epub 2022 Jan 4. J Periodontal Res. 2022. PMID: 34984683 Free PMC article.
-
Identification and characterization of a novel adiponectin receptor agonist adipo anti-inflammation agonist and its anti-inflammatory effects in vitro and in vivo.Br J Pharmacol. 2021 Jan;178(2):280-297. doi: 10.1111/bph.15277. Epub 2020 Dec 18. Br J Pharmacol. 2021. PMID: 32986862 Free PMC article.
-
Adiponectin and adiponectin receptors.Endocr Rev. 2005 May;26(3):439-51. doi: 10.1210/er.2005-0005. Endocr Rev. 2005. PMID: 15897298 Review.
-
Adiponectin receptors: a review of their structure, function and how they work.Best Pract Res Clin Endocrinol Metab. 2014 Jan;28(1):15-23. doi: 10.1016/j.beem.2013.09.003. Epub 2013 Sep 15. Best Pract Res Clin Endocrinol Metab. 2014. PMID: 24417942 Review.
Cited by
-
Adipokines in the Crosstalk between Adipose Tissues and Other Organs: Implications in Cardiometabolic Diseases.Biomedicines. 2024 Sep 19;12(9):2129. doi: 10.3390/biomedicines12092129. Biomedicines. 2024. PMID: 39335642 Free PMC article. Review.
References
-
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, … Kadowaki T (2011). Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab, 13(4), 401–412. doi:S1550-4131(11)00083-0 [pii] 10.1016/j.cmet.2011.02.010 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

