This is a preprint.
Macrophage-induced reduction of bacteriophage density limits the efficacy of in vivo pulmonary phage therapy
- PMID: 38293203
- PMCID: PMC10827109
- DOI: 10.1101/2024.01.16.575879
Macrophage-induced reduction of bacteriophage density limits the efficacy of in vivo pulmonary phage therapy
Abstract
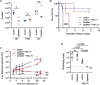
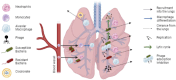
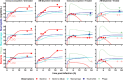
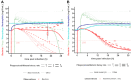
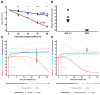
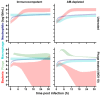
The rise of antimicrobial resistance has led to renewed interest in evaluating phage therapy. In murine models highly effective treatment of acute pneumonia caused by Pseudomonas aeruginosa relies on the synergistic antibacterial activity of bacteriophages with neutrophils. Here, we show that depletion of alveolar macrophages (AM) shortens the survival of mice without boosting the P. aeruginosa load in the lungs. Unexpectedly, upon bacteriophage treatment, pulmonary levels of P. aeruginosa were significantly lower in AM-depleted than in immunocompetent mice. To explore potential mechanisms underlying the benefit of AM-depletion in treated mice, we developed a mathematical model of phage, bacteria, and innate immune system dynamics. Simulations from the model fitted to data suggest that AM reduce bacteriophage density in the lungs. We experimentally confirmed that the in vivo decay of bacteriophage is faster in immunocompetent compared to AM-depleted animals. These findings demonstrate the involvement of feedback between bacteriophage, bacteria, and the immune system in shaping the outcomes of phage therapy in clinical settings.
Keywords: Antimicrobial resistance; Bacterial infection; Innate immunity; Mathematical model; Pneumonia.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures
Similar articles
-
Metapopulation model of phage therapy of an acute Pseudomonas aeruginosa lung infection.mSystems. 2024 Oct 22;9(10):e0017124. doi: 10.1128/msystems.00171-24. Epub 2024 Sep 4. mSystems. 2024. PMID: 39230264 Free PMC article.
-
Inhaled bacteriophage therapy in a porcine model of pneumonia caused by Pseudomonas aeruginosa during mechanical ventilation.Br J Pharmacol. 2021 Sep;178(18):3829-3842. doi: 10.1111/bph.15526. Epub 2021 Jul 9. Br J Pharmacol. 2021. PMID: 33974271
-
Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen.Cell Host Microbe. 2017 Jul 12;22(1):38-47.e4. doi: 10.1016/j.chom.2017.06.018. Cell Host Microbe. 2017. PMID: 28704651
-
Bacteriophage-based therapy in cystic fibrosis-associated Pseudomonas aeruginosa infections: rationale and current status.Drug Des Devel Ther. 2015 Jul 16;9:3653-63. doi: 10.2147/DDDT.S53123. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26213462 Free PMC article. Review.
-
Fitness Trade-Offs Resulting from Bacteriophage Resistance Potentiate Synergistic Antibacterial Strategies.Infect Immun. 2020 Jun 22;88(7):e00926-19. doi: 10.1128/IAI.00926-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32094257 Free PMC article. Review.
References
-
- WHO, O. (2017). One health. World Health Organ.
Publication types
LinkOut - more resources
Full Text Sources
