Terpenes and cannabidiol against human corona and influenza viruses-Anti-inflammatory and antiviral in vitro evaluation
- PMID: 38318445
- PMCID: PMC10840330
- DOI: 10.1016/j.btre.2024.e00829
Terpenes and cannabidiol against human corona and influenza viruses-Anti-inflammatory and antiviral in vitro evaluation
Abstract
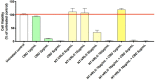
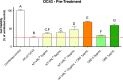
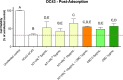
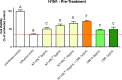
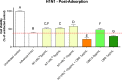
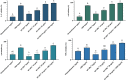
The activity of the terpenes and Cannabidiol (CBD) against human coronavirus (HCoV) strain OC43 and influenza A (H1N1) was evaluated in human lung fibroblasts (MRC-5 cells). Also, we examined whether these ingredients inhibit pro-inflammatory cytokines in peripheral blood mononuclear cells (PBMC). The tested preparations exhibited both anti-inflammatory and antiviral effects. The combination of terpenes was effective against both HCoV-OC43 and influenza A (H1N1) virus. The addition of CBD improved the antiviral activity in some, but not all cases. This variation in activity may suggest an antiviral mechanism. In addition, there was a strong correlation between the quantitative results from a cell-viability assay and the cytopathic effect after 72 h, as observed under a microscope. The anti-inflammatory properties of terpenes were demonstrated using a pro-inflammatory cytokine-inhibition assay, which revealed significant cytokine inhibition and enhanced by the addition of CBD.
Keywords: CBD; Coronavirus; Cytokine storm; Influenza virus; Terpenes.
© 2024 The Authors.
Conflict of interest statement
Lior Chatow, Nadav Eyal, Silvia Ramirez and Adi Nudel are employees of Eybna Technologies, a company that manufactures terpene-based formulations. Iris Nesher and Richard Boxer are advisors of Eybna Technologies.
Figures



Similar articles
-
In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229.Life (Basel). 2021 Mar 29;11(4):290. doi: 10.3390/life11040290. Life (Basel). 2021. PMID: 33805385 Free PMC article.
-
Broad antiviral and anti-inflammatory activity of Qingwenjiere mixture against SARS-CoV-2 and other human coronavirus infections.Phytomedicine. 2021 Dec;93:153808. doi: 10.1016/j.phymed.2021.153808. Epub 2021 Oct 18. Phytomedicine. 2021. PMID: 34753027 Free PMC article.
-
Cannabidiol: Influence on B Cells, Peripheral Blood Mononuclear Cells, and Peripheral Blood Mononuclear Cell/Rheumatoid Arthritis Synovial Fibroblast Cocultures.Cannabis Cannabinoid Res. 2023 Apr;8(2):321-334. doi: 10.1089/can.2021.0241. Epub 2022 Aug 3. Cannabis Cannabinoid Res. 2023. PMID: 35920857
-
[Cytokine storm in avian influenza].Mikrobiyol Bul. 2008 Apr;42(2):365-80. Mikrobiyol Bul. 2008. PMID: 18697437 Review. Turkish.
-
An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses.Microb Pathog. 2021 Mar;152:104620. doi: 10.1016/j.micpath.2020.104620. Epub 2020 Nov 16. Microb Pathog. 2021. PMID: 33212200 Free PMC article. Review.
Cited by
-
Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3.Int J Mol Sci. 2024 Aug 3;25(15):8480. doi: 10.3390/ijms25158480. Int J Mol Sci. 2024. PMID: 39126048 Free PMC article.
-
Antiviral effect of cannabidiol on K18-hACE2 transgenic mice infected with SARS-CoV-2.J Cell Mol Med. 2024 Sep;28(17):e70030. doi: 10.1111/jcmm.70030. J Cell Mol Med. 2024. PMID: 39267200 Free PMC article.
References
-
- Zamora A., Edmonds J.H., Reynolds M.J., Khromykh A.A., Ralph S.J. The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology. 2016;495:18–32. - PubMed
-
- Bicchi C., Rubiolo P., Ballero M., Sanna C., Matteodo M., Esposito F., et al. HIV-1-inhibiting activity of the essential oil of Ridolfia segetum and Oenanthe crocata. Planta Med. 2009;75(12):1331–1335. - PubMed
LinkOut - more resources
Full Text Sources
