Advances in the Use of Conducting Polymers for Healthcare Monitoring
- PMID: 38338846
- PMCID: PMC10855550
- DOI: 10.3390/ijms25031564
Advances in the Use of Conducting Polymers for Healthcare Monitoring
Abstract
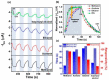
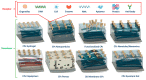
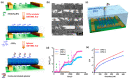
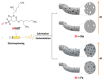
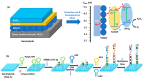
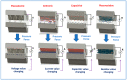
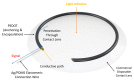
Conducting polymers (CPs) are an innovative class of materials recognized for their high flexibility and biocompatibility, making them an ideal choice for health monitoring applications that require flexibility. They are active in their design. Advances in fabrication technology allow the incorporation of CPs at various levels, by combining diverse CPs monomers with metal particles, 2D materials, carbon nanomaterials, and copolymers through the process of polymerization and mixing. This method produces materials with unique physicochemical properties and is highly customizable. In particular, the development of CPs with expanded surface area and high conductivity has significantly improved the performance of the sensors, providing high sensitivity and flexibility and expanding the range of available options. However, due to the morphological diversity of new materials and thus the variety of characteristics that can be synthesized by combining CPs and other types of functionalities, choosing the right combination for a sensor application is difficult but becomes important. This review focuses on classifying the role of CP and highlights recent advances in sensor design, especially in the field of healthcare monitoring. It also synthesizes the sensing mechanisms and evaluates the performance of CPs on electrochemical surfaces and in the sensor design. Furthermore, the applications that can be revolutionized by CPs will be discussed in detail.
Keywords: conducting polymers; human health monitoring; sensor technology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Conductive polymer-based sensors for biomedical applications.Biosens Bioelectron. 2011 Jan 15;26(5):1825-32. doi: 10.1016/j.bios.2010.09.046. Epub 2010 Oct 1. Biosens Bioelectron. 2011. PMID: 21030240
-
Conducting Polymers and Their Applications in Diabetes Management.Sensors (Basel). 2016 Oct 26;16(11):1787. doi: 10.3390/s16111787. Sensors (Basel). 2016. PMID: 27792179 Free PMC article. Review.
-
Conducting polymers for electrochemical DNA sensing.Biomaterials. 2009 Apr;30(11):2132-48. doi: 10.1016/j.biomaterials.2008.12.065. Epub 2009 Jan 14. Biomaterials. 2009. PMID: 19147223 Review.
-
Conducting polymer nanomaterials: electrosynthesis and applications.Chem Soc Rev. 2009 Aug;38(8):2397-409. doi: 10.1039/b816681c. Epub 2009 Apr 3. Chem Soc Rev. 2009. PMID: 19623357 Review.
-
Recent Advances of Conducting Polymers and Their Composites for Electrochemical Biosensing Applications.J Funct Biomater. 2020 Sep 25;11(4):71. doi: 10.3390/jfb11040071. J Funct Biomater. 2020. PMID: 32992861 Free PMC article. Review.
Cited by
-
Organic and Metal-Organic Polymer-Based Catalysts-Enfant Terrible Companions or Good Assistants?Molecules. 2024 Sep 29;29(19):4623. doi: 10.3390/molecules29194623. Molecules. 2024. PMID: 39407552 Free PMC article. Review.
-
Exploring Electrochemical Sensing for Fungicide Detection: Utilization of Newly Synthesized Oligomers.ACS Omega. 2024 Aug 16;9(34):36622-36634. doi: 10.1021/acsomega.4c04959. eCollection 2024 Aug 27. ACS Omega. 2024. PMID: 39220534 Free PMC article.
-
Comparative Analysis of the Mechanical Properties and Biocompatibility between CAD/CAM and Conventional Polymers Applied in Prosthetic Dentistry.Polymers (Basel). 2024 Mar 22;16(7):877. doi: 10.3390/polym16070877. Polymers (Basel). 2024. PMID: 38611135 Free PMC article. Review.
References
-
- Wang M., Tang H.X., Cai H.J., Wu H., Shen B.J., Guo Y.S. Construction, mechanism and prospective of conductive polymer composites with multiple interfaces for electromagnetic interference shielding: A review. Carbon. 2021;177:377–402. doi: 10.1016/j.carbon.2021.02.047. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

