Genetic disruption of dopamine β-hydroxylase dysregulates innate responses to predator odor in mice
- PMID: 38371489
- PMCID: PMC10873756
- DOI: 10.1016/j.ynstr.2024.100612
Genetic disruption of dopamine β-hydroxylase dysregulates innate responses to predator odor in mice
Abstract
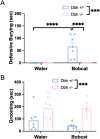
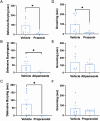
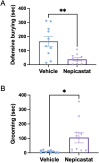
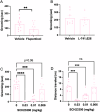
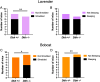
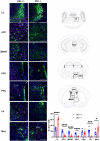
In rodents, exposure to predator odors such as cat urine acts as a severe stressor that engages innate defensive behaviors critical for survival in the wild. The neurotransmitters norepinephrine (NE) and dopamine (DA) modulate anxiety and predator odor responses, and we have shown previously that dopamine β-hydroxylase knockout (Dbh -/-), which reduces NE and increases DA in mouse noradrenergic neurons, disrupts innate behaviors in response to mild stressors such as novelty. We examined the consequences of Dbh knockout on responses to predator odor (bobcat urine) and compared them to Dbh-competent littermate controls. Over the first 10 min of predator odor exposure, controls exhibited robust defensive burying behavior, whereas Dbh -/- mice showed high levels of grooming. Defensive burying was potently suppressed in controls by drugs that reduce NE transmission, while excessive grooming in Dbh -/- mice was blocked by DA receptor antagonism. In response to a cotton square scented with a novel "neutral" odor (lavender), most control mice shredded the material, built a nest, and fell asleep within 90 min. Dbh -/- mice failed to shred the lavender-scented nestlet, but still fell asleep. In contrast, controls sustained high levels of arousal throughout the predator odor test and did not build nests, while Dbh -/- mice were asleep by the 90-min time point, often in shredded bobcat urine-soaked nesting material. Compared with controls exposed to predator odor, Dbh -/- mice demonstrated decreased c-fos induction in the anterior cingulate cortex, lateral septum, periaqueductal gray, and bed nucleus of the stria terminalis, but increased c-fos in the locus coeruleus and medial amygdala. These data indicate that relative ratios of central NE and DA signaling coordinate the type and valence of responses to predator odor.
Keywords: Dopamine; Dopamine β-hydroxylase; Norepinephrine; Predator; Toxoplasma gondii.
© 2024 The Authors.
Conflict of interest statement
DW is co-inventor on a patent concerning the use of selective dopamine β-hydroxylase inhibitors for the treatment of cocaine dependence (US-2010-0105,748-A1; “Methods and Compositions for Treatment of Drug Addiction”). The other authors declare no conflicts of interest.
Figures
Update of
-
Genetic disruption of dopamine β-hydroxylase dysregulates innate responses to predator odor in mice.bioRxiv [Preprint]. 2024 Jan 27:2023.06.21.545975. doi: 10.1101/2023.06.21.545975. bioRxiv. 2024. Update in: Neurobiol Stress. 2024 Feb 02;29:100612. doi: 10.1016/j.ynstr.2024.100612 PMID: 38234825 Free PMC article. Updated. Preprint.
Similar articles
-
Genetic disruption of dopamine β-hydroxylase dysregulates innate responses to predator odor in mice.bioRxiv [Preprint]. 2024 Jan 27:2023.06.21.545975. doi: 10.1101/2023.06.21.545975. bioRxiv. 2024. Update in: Neurobiol Stress. 2024 Feb 02;29:100612. doi: 10.1016/j.ynstr.2024.100612 PMID: 38234825 Free PMC article. Updated. Preprint.
-
Noradrenergic circuits in the forebrain control affective responses to novelty.Psychopharmacology (Berl). 2020 Nov;237(11):3337-3355. doi: 10.1007/s00213-020-05615-8. Epub 2020 Jul 28. Psychopharmacology (Berl). 2020. PMID: 32821984 Free PMC article.
-
Norepinephrine and dopamine contribute to distinct repetitive behaviors induced by novel odorant stress in male and female mice.Horm Behav. 2022 Aug;144:105205. doi: 10.1016/j.yhbeh.2022.105205. Epub 2022 Jun 1. Horm Behav. 2022. PMID: 35660247 Free PMC article.
-
Influence of Cat Odor on Reproductive Behavior and Physiology in the House Mouse: (Mus Musculus).In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 14. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 14. PMID: 24830030 Free Books & Documents. Review.
-
Olfactory systems and neural circuits that modulate predator odor fear.Front Behav Neurosci. 2014 Mar 11;8:72. doi: 10.3389/fnbeh.2014.00072. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24653685 Free PMC article. Review.
Cited by
-
The functional role of locus coeruleus microglia in the female stress response.bioRxiv [Preprint]. 2024 Sep 11:2024.01.10.575076. doi: 10.1101/2024.01.10.575076. bioRxiv. 2024. PMID: 38260568 Free PMC article. Preprint.
References
-
- Aghajanian G.K., VanderMaelen C.P. Alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. - PubMed
-
- Berridge K.C., Aldridge J.W. Super-stereotypy I: enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse. 2000;37:194–204. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

