Inhibition of RANKL improves the skeletal phenotype of adenine-induced chronic kidney disease in mice
- PMID: 38505524
- PMCID: PMC10945718
- DOI: 10.1093/jbmrpl/ziae004
Inhibition of RANKL improves the skeletal phenotype of adenine-induced chronic kidney disease in mice
Erratum in
-
Correction to: Inhibition of RANKL improves the skeletal phenotype of adenine-induced chronic kidney disease in mice.JBMR Plus. 2024 May 8;8(6):ziae059. doi: 10.1093/jbmrpl/ziae059. eCollection 2024 Jun. JBMR Plus. 2024. PMID: 38721044 Free PMC article.
Abstract
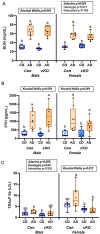
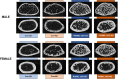
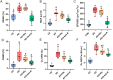
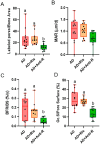
Skeletal fragility and high fracture rates are common in CKD. A key component of bone loss in CKD with secondary hyperparathyroidism is high bone turnover and cortical bone deterioration through both cortical porosity and cortical thinning. We hypothesized that RANKL drives high bone resorption within cortical bone leading to the development of cortical porosity in CKD (study 1) and that systemic inhibition of RANKL would mitigate the skeletal phenotype of CKD (study 2). In study 1, we assessed the skeletal properties of male and female Dmp1-cre RANKLfl/fl (cKO) and control genotype (Ranklfl/fl; Con) mice after 10 wk of adenine-induced CKD (AD; 0.2% dietary adenine). All AD mice regardless of sex or genotype had elevated blood urea nitrogen and high PTH. Con AD mice in both sexes had cortical porosity and lower cortical thickness as well as high osteoclast-covered trabecular surfaces and higher bone formation rate. cKO mice had preserved cortical bone microarchitecture despite high circulating PTH as well as no CKD-induced increases in osteoclasts. In study 2, male mice with established AD CKD were either given a single injection of an anti-RANKL antibody (5 mg/kg) 8 wk post-induction of CKD or subjected to 3×/wk dosing with risedronate (1.2 μg/kg) for 4 wk. Anti-RANKL treatment significantly reduced bone formation rate as well as osteoclast surfaces at both trabecular and cortical pore surfaces; risedronate treatment had little effect on these bone parameters. In conclusion, these studies demonstrate that bone-specific RANKL is critical for the development of high bone formation/high osteoclasts and cortical bone loss in CKD with high PTH. Additionally, systemic anti-RANKL ligand therapy in established CKD may help prevent the propagation of cortical bone loss via suppression of bone turnover.
Keywords: PTH; RANKL; chronic kidney disease; cortical porosity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
M.R.A. consults for and receives financial support from MBX Biosciences as well as receiving book royalties from Elsevier. All other authors have nothing to disclose.
Figures


Similar articles
-
Strain-specific alterations in the skeletal response to adenine-induced chronic kidney disease are associated with differences in parathyroid hormone levels.Bone. 2021 Jul;148:115963. doi: 10.1016/j.bone.2021.115963. Epub 2021 Apr 17. Bone. 2021. PMID: 33878503 Free PMC article.
-
Elevations in Cortical Porosity Occur Prior to Significant Rise in Serum Parathyroid Hormone in Young Female Mice with Adenine-Induced CKD.Calcif Tissue Int. 2020 Apr;106(4):392-400. doi: 10.1007/s00223-019-00642-w. Epub 2019 Dec 12. Calcif Tissue Int. 2020. PMID: 31832725 Free PMC article.
-
Combining raloxifene and mechanical loading improves bone composition and mechanical properties in a murine model of chronic kidney disease (CKD).Bone. 2024 Jun;183:117089. doi: 10.1016/j.bone.2024.117089. Epub 2024 Apr 3. Bone. 2024. PMID: 38575047
-
The skeleton in primary hyperparathyroidism: a review focusing on bone remodeling, structure, mass, and fracture.APMIS Suppl. 2001;(102):1-52. APMIS Suppl. 2001. PMID: 11419022 Review.
-
Systemic Activation of Activin A Signaling Causes Chronic Kidney Disease-Mineral Bone Disorder.Int J Mol Sci. 2018 Aug 23;19(9):2490. doi: 10.3390/ijms19092490. Int J Mol Sci. 2018. PMID: 30142896 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

