Sex-based differences in growth-related IGF1 signaling in response to PAPP-A2 deficiency: comparative effects of rhGH, rhIGF1 and rhPAPP-A2 treatments
- PMID: 38589872
- PMCID: PMC11000399
- DOI: 10.1186/s13293-024-00603-5
Sex-based differences in growth-related IGF1 signaling in response to PAPP-A2 deficiency: comparative effects of rhGH, rhIGF1 and rhPAPP-A2 treatments
Abstract
Background: Children with pregnancy-associated plasma protein-A2 (PAPP-A2) mutations resulting in low levels of bioactive insulin-like growth factor-1 (IGF1) and progressive postnatal growth retardation have improved growth velocity and height following recombinant human (rh)IGF1 treatment. The present study aimed to evaluate whether Pappa2 deficiency and pharmacological manipulation of GH/IGF1 system are associated with sex-specific differences in growth-related signaling pathways.
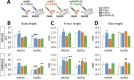
Methods: Plasma, hypothalamus, pituitary gland and liver of Pappa2ko/ko mice of both sexes, showing reduced skeletal growth, and liver of these mice treated with rhGH, rhIGF1 and rhPAPP-A2 from postnatal day (PND) 5 to PND35 were analyzed.
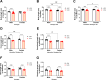
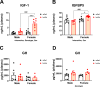
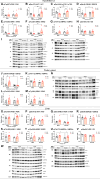
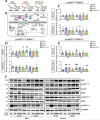
Results: Reduced body and femur length of Pappa2ko/ko mice was associated with increases in: (1) components of IGF1 ternary complexes (IGF1, IGFBP5/Igfbp5, Igfbp3, Igfals) in plasma, hypothalamus and/or liver; and (2) key signaling regulators (phosphorylated PI3K, AKT, mTOR, GSK3β, ERK1/2 and AMPKα) in hypothalamus, pituitary gland and/or liver, with Pappa2ko/ko females having a more prominent effect. Compared to rhGH and rhIGF1, rhPAPP-A2 specifically induced: (1) increased body and femur length, and reduced plasma total IGF1 and IGFBP5 concentrations in Pappa2ko/ko females; and (2) increased Igf1 and Igf1r levels and decreased Ghr, Igfbp3 and Igfals levels in the liver of Pappa2ko/ko females. These changes were accompanied by lower phospho-STAT5, phospho-AKT and phospho-ERK2 levels and higher phospho-AMPK levels in the liver of Pappa2ko/ko females.
Conclusions: Sex-specific differences in IGF1 system and signaling pathways are associated with Pappa2 deficiency, pointing to rhPAPP-A2 as a promising drug to alleviate postnatal growth retardation underlying low IGF1 bioavailability in a female-specific manner.
Keywords: AMPK; GH; Growth; Hypothalamus; IGF1; Liver; PI3K; Pappalysin-2; Pituitary gland; Sexual dimorphism; mTOR.
Plain language summary
Understanding the physiological role of pregnancy-associated plasma protein-A2 (PAPP-A2), a proteinase involved in the insulin-like growth factor-1 (IGF1) availability to regulate growth, could provide insight into new treatments for patients with short stature and skeletal abnormalities. Although progressive postnatal growth retardation in patients with PAPP-A2 mutations can differ between males and females, we do not know the underlying differences in IGF1 system and signaling, and their response to treatment that contribute to growth improvement. The present study examines whether Pappa2 deficiency and pharmacological administration of rhGH, rhIGF1 and rhPAPP-A2 are associated with sex-specific differences in IGF1 ternary complexes and IGF1 signaling pathways. Reduced body and femur length of Pappa2-deficient mice was associated with sex- and tissue-specific alteration of IGF ternary/binary complexes and IGF1 signaling pathways. rhPAPP-A2 treatment induced female-specific increase in body and femur length and reduction in IGF ternary/binary complexes through STAT5-AKT-ERK2-AMPK signaling pathways in liver. The involvement of PAPP-A2 in sex-based growth physiology supports the use of promising drugs to alleviate postnatal growth retardation underlying low IGF1 bioavailability in a female-specific manner.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Short stature with low insulin-like growth factor 1 availability due to pregnancy-associated plasma protein A2 deficiency in a Saudi family.Clin Genet. 2021 Nov;100(5):601-606. doi: 10.1111/cge.14030. Epub 2021 Jul 22. Clin Genet. 2021. PMID: 34272725
-
Recombinant IGF-1 Induces Sex-Specific Changes in Bone Composition and Remodeling in Adult Mice with Pappa2 Deficiency.Int J Mol Sci. 2021 Apr 14;22(8):4048. doi: 10.3390/ijms22084048. Int J Mol Sci. 2021. PMID: 33919940 Free PMC article.
-
Metabolomics changes in patients with PAPP-A2 deficiency in response to rhIGF1 treatment.Growth Horm IGF Res. 2018 Oct-Dec;42-43:28-31. doi: 10.1016/j.ghir.2018.08.002. Epub 2018 Aug 12. Growth Horm IGF Res. 2018. PMID: 30119035
-
Genetic disorders of GH action pathway.Growth Horm IGF Res. 2018 Feb;38:19-23. doi: 10.1016/j.ghir.2017.12.004. Epub 2017 Dec 12. Growth Horm IGF Res. 2018. PMID: 29249625 Review.
-
Novel Modulators of the Growth Hormone - Insulin-Like Growth Factor Axis: Pregnancy-Associated Plasma Protein-A2 and Stanniocalcin-2.J Clin Res Pediatr Endocrinol. 2017 Dec 30;9(Suppl 2):1-8. doi: 10.4274/jcrpe.2017.S001. Epub 2017 Dec 27. J Clin Res Pediatr Endocrinol. 2017. PMID: 29280739 Free PMC article. Review.
References
-
- Dauber A, Muñoz-Calvo MT, Barrios V, Domené HM, Kloverpris S, Serra-Juhé C, Desikan V, Pozo J, Muzumdar R, Martos-Moreno GÁ, Hawkins F, Jasper HG, Conover CA, Frystyk J, Yakar S, Hwa V, Chowen JA, Oxvig C, Rosenfeld RG, Pérez-Jurado LA, Argente J. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med. 2016;8(4):363–74. doi: 10.15252/emmm.201506106. - DOI - PMC - PubMed
-
- Babiker A, Al Noaim K, Al Swaid A, Alfadhel M, Deeb A, Martín-Rivada Á, Barrios V, Pérez-Jurado LA, Alfares A, Al Alwan I, Argente J. Short stature with low insulin-like growth factor 1 availability due to pregnancy-associated plasma protein A2 deficiency in a Saudi family. Clin Genet. 2021;100(5):601–6. doi: 10.1111/cge.14030. - DOI - PubMed
-
- Muñoz-Calvo MT, Barrios V, Pozo J, Chowen JA, Martos-Moreno GÁ, Hawkins F, Dauber A, Domené HM, Yakar S, Rosenfeld RG, Pérez-Jurado LA, Oxvig C, Frystyk J, Argente J. Treatment with recombinant human insulin-like Growth Factor-1 improves growth in patients with PAPP-A2 Deficiency. J Clin Endocrinol Metab. 2016;101(11):3879–83. doi: 10.1210/jc.2016-2751. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

