Advances in cell-based delivery of oncolytic viruses as therapy for lung cancer
- PMID: 38596310
- PMCID: PMC10976516
- DOI: 10.1016/j.omton.2024.200788
Advances in cell-based delivery of oncolytic viruses as therapy for lung cancer
Erratum in
-
Erratum: Advances in cell-based delivery of oncolytic viruses as therapy for lung cancer.Mol Ther Oncol. 2024 Jul 23;32(3):200848. doi: 10.1016/j.omton.2024.200848. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39135745 Free PMC article.
Abstract
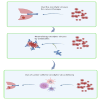
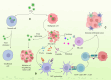
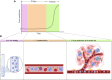
Lung cancer's intractability is enhanced by its frequent resistance to (chemo)therapy and often high relapse rates that make it the leading cause of cancer death worldwide. Improvement of therapy efficacy is a crucial issue that might lead to a significant advance in the treatment of lung cancer. Oncolytic viruses are desirable combination partners in the developing field of cancer immunotherapy due to their direct cytotoxic effects and ability to elicit an immune response. Systemic oncolytic virus administration through intravenous injection should ideally lead to the highest efficacy in oncolytic activity. However, this is often hampered by the prevalence of host-specific, anti-viral immune responses. One way to achieve more efficient systemic oncolytic virus delivery is through better protection against neutralization by several components of the host immune system. Carrier cells, which can even have innate tumor tropism, have shown their appropriateness as effective vehicles for systemic oncolytic virus infection through circumventing restrictive features of the immune system and can warrant oncolytic virus delivery to tumors. In this overview, we summarize promising results from studies in which carrier cells have shown their usefulness for improved systemic oncolytic virus delivery and better oncolytic virus therapy against lung cancer.
Keywords: antibody neutralization; cell-mediated carrier; lung cancer; oncolytic viral therapy; _target delivery.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest. This work was supported in part by TÜSEB grant no. 2022-B-01-16483 (to R.M.), for the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures

Similar articles
-
Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1.J Immunother Cancer. 2019 Aug 10;7(1):214. doi: 10.1186/s40425-019-0682-1. J Immunother Cancer. 2019. PMID: 31399043 Free PMC article.
-
Generation of novel oncolytic vaccinia virus with improved intravenous efficacy through protection against complement-mediated lysis and evasion of neutralization by vaccinia virus-specific antibodies.J Immunother Cancer. 2023 Jan;11(1):e006024. doi: 10.1136/jitc-2022-006024. J Immunother Cancer. 2023. PMID: 36717184 Free PMC article.
-
Cell carriers for oncolytic viruses: current challenges and future directions.Oncolytic Virother. 2013 Oct 9;2:47-56. doi: 10.2147/OV.S36623. eCollection 2013. Oncolytic Virother. 2013. PMID: 27512657 Free PMC article. Review.
-
Multidirectional Strategies for _targeted Delivery of Oncolytic Viruses by Tumor Infiltrating Immune Cells.Pharmacol Res. 2020 Nov;161:105094. doi: 10.1016/j.phrs.2020.105094. Epub 2020 Aug 12. Pharmacol Res. 2020. PMID: 32795509 Review.
-
PolySia-Specific Re_targeting of Oncolytic Viruses Triggers Tumor-Specific Immune Responses and Facilitates Therapy of Disseminated Lung Cancer.Cancer Immunol Res. 2015 Jul;3(7):751-63. doi: 10.1158/2326-6066.CIR-14-0124-T. Epub 2015 Feb 19. Cancer Immunol Res. 2015. PMID: 25701327
Cited by
-
Enhancing cancer therapy: the integration of oncolytic virus therapy with diverse treatments.Cancer Cell Int. 2024 Jul 11;24(1):242. doi: 10.1186/s12935-024-03424-z. Cancer Cell Int. 2024. PMID: 38992667 Free PMC article. Review.
References
-
- Dorer D.E., Nettelbeck D.M. _targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv. Drug Deliv. Rev. 2009;61:554–571. - PubMed
-
- Hadryś A., Sochanik A., McFadden G., Jazowiecka-Rakus J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur. J. Pharmacol. 2020;874 - PubMed
-
- Mountzios G., Remon J., Hendriks L.E.L., García-Campelo R., Rolfo C., Van Schil P., Forde P.M., Besse B., Subbiah V., Reck M., et al. Immune-checkpoint inhibition for resectable non-small-cell lung cancer — opportunities and challenges. Nat. Rev. Clin. Oncol. 2023;20:664–677. - PubMed
-
- Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
