Role of protein Post-translational modifications in enterovirus infection
- PMID: 38596371
- PMCID: PMC11002909
- DOI: 10.3389/fmicb.2024.1341599
Role of protein Post-translational modifications in enterovirus infection
Abstract
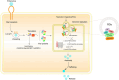
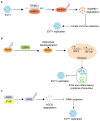
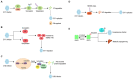
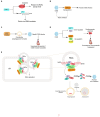
Enteroviruses (EVs) are the main cause of a number of neurological diseases. Growing evidence has revealed that successful infection with enteroviruses is highly dependent on the host machinery, therefore, host proteins play a pivotal role in viral infections. Both host and viral proteins can undergo post-translational modification (PTM) which can regulate protein activity, stability, solubility and interactions with other proteins; thereby influencing various biological processes, including cell metabolism, metabolic, signaling pathways, cell death, and cancer development. During viral infection, both host and viral proteins regulate the viral life cycle through various PTMs and different mechanisms, including the regulation of host cell entry, viral protein synthesis, genome replication, and the antiviral immune response. Therefore, protein PTMs play important roles in EV infections. Here, we review the role of various host- and virus-associated PTMs during enterovirus infection.
Keywords: enterovirus infection; enterovirus life cycle; host factors; pathogenesis; post-translation modification.
Copyright © 2024 Zhao, Hu, Zhao, Liu, Ma, Chen and Xing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of Host-Mediated Post-Translational Modifications (PTMs) in RNA Virus Pathogenesis.Int J Mol Sci. 2020 Dec 30;22(1):323. doi: 10.3390/ijms22010323. Int J Mol Sci. 2020. PMID: 33396899 Free PMC article. Review.
-
SUMO Modification Stabilizes Enterovirus 71 Polymerase 3D To Facilitate Viral Replication.J Virol. 2016 Nov 14;90(23):10472-10485. doi: 10.1128/JVI.01756-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630238 Free PMC article.
-
Essential Role of Enterovirus 2A Protease in Counteracting Stress Granule Formation and the Induction of Type I Interferon.J Virol. 2019 May 1;93(10):e00222-19. doi: 10.1128/JVI.00222-19. Print 2019 May 15. J Virol. 2019. PMID: 30867299 Free PMC article.
-
Role of Post-translational Modifications in Influenza A Virus Life Cycle and Host Innate Immune Response.Front Microbiol. 2020 Sep 4;11:517461. doi: 10.3389/fmicb.2020.517461. eCollection 2020. Front Microbiol. 2020. PMID: 33013775 Free PMC article. Review.
-
Post-translational Modification-Based Regulation of HIV Replication.Front Microbiol. 2018 Sep 11;9:2131. doi: 10.3389/fmicb.2018.02131. eCollection 2018. Front Microbiol. 2018. PMID: 30254620 Free PMC article. Review.
Cited by
-
Application of Proteomics Technology Based on LC-MS Combined with Western Blotting and Co-IP in Antiviral Innate Immunity.Methods Mol Biol. 2025;2854:93-106. doi: 10.1007/978-1-0716-4108-8_11. Methods Mol Biol. 2025. PMID: 39192122
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

