Membranes on the move: The functional role of the extracellular vesicle membrane for contact-dependent cellular signalling
- PMID: 38649339
- PMCID: PMC11035383
- DOI: 10.1002/jev2.12436
Membranes on the move: The functional role of the extracellular vesicle membrane for contact-dependent cellular signalling
Abstract
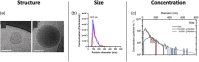
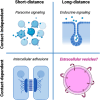
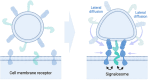
Extracellular vesicles (EVs), lipid-enclosed structures released by virtually all life forms, have gained significant attention due to their role in intercellular and interorganismal communication. Despite their recognized importance in disease processes and therapeutic applications, fundamental questions about their primary function remain. Here, we propose a different perspective on the primary function of EVs, arguing that they serve as essential elements providing membrane area for long-distance, contact-dependent cellular communication based on protein-protein interaction. While EVs have been recognized as carriers of genetic information, additional unique advantages that they could provide for cellular communication remain unclear. Here, we introduce the concept that the substantial membrane area provided by EVs allows for membrane contact-dependent interactions that could be central to their function. This membrane area enables the lateral diffusion and sorting of membrane ligands like proteins, polysaccharides or lipids in two dimensions, promoting avidity-driven effects and assembly of co-stimulatory architectures at the EV-cell interface. The concept of vesicle-induced receptor sequestration (VIRS), for example, describes how EVs confine and focus receptors at the EV contact site, promoting a dense local concentration of receptors into signalosomes. This process can increase the signalling strength of EV-presented ligands by 10-1000-fold compared to their soluble counterparts. The speculations in this perspective advance our understanding of EV-biology and have critical implications for EV-based applications and therapeutics. We suggest a shift in perspective from viewing EVs merely as transporters of relevant nucleic acids and proteins to considering their unique biophysical properties as presentation platforms for long-distance, contact-dependent signalling. We therefore highlight the functional role of the EV membrane rather than their content. We further discuss how this signalling mechanism might be exploited by virus-transformed or cancer cells to enhance immune-evasive mechanisms.
Keywords: contact‐dependent signalling; extracellular vesicles; immunotherapy; membrane biology; signalosomes.
© 2024 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Vesicle Induced Receptor Sequestration: Mechanisms behind Extracellular Vesicle-Based Protein Signaling.Adv Sci (Weinh). 2022 May;9(13):e2200201. doi: 10.1002/advs.202200201. Epub 2022 Mar 1. Adv Sci (Weinh). 2022. PMID: 35233981 Free PMC article.
-
From intra- to extracellular vesicles: extracellular vesicles in developmental signalling.Essays Biochem. 2018 May 15;62(2):215-223. doi: 10.1042/EBC20180001. Print 2018 May 15. Essays Biochem. 2018. PMID: 29765007 Review.
-
Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles.Int J Nanomedicine. 2021 May 18;16:3357-3383. doi: 10.2147/IJN.S310357. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34040369 Free PMC article. Review.
-
Extracellular signals regulate the biogenesis of extracellular vesicles.Biol Res. 2022 Nov 26;55(1):35. doi: 10.1186/s40659-022-00405-2. Biol Res. 2022. PMID: 36435789 Free PMC article. Review.
-
Intercellular Molecular Transfer Mediated by Extracellular Vesicles in Cancer.Results Probl Cell Differ. 2024;73:327-352. doi: 10.1007/978-3-031-62036-2_14. Results Probl Cell Differ. 2024. PMID: 39242385 Review.
Cited by
-
Harnessing extracellular vesicle heterogeneity for diagnostic and therapeutic applications.Nat Nanotechnol. 2024 Oct 28. doi: 10.1038/s41565-024-01774-3. Online ahead of print. Nat Nanotechnol. 2024. PMID: 39468355 Review.
References
-
- Audo, R. , Calmon‐Hamaty, F. , Combe, B. , Hahne, M. , & Morel, J. (2012). Dual effects of soluble FasL and membrane bound FasL on fibroblast‐like synoviocytes cells from rheumatoid arthritis patients. Annals of the Rheumatic Diseases, 71, A86–A86. 10.1136/annrheumdis-2011-201239.3 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

