Intravenous immunoglobulins for the treatment of prolonged COVID-19 in immunocompromised patients: a brief report
- PMID: 38707896
- PMCID: PMC11069322
- DOI: 10.3389/fimmu.2024.1399180
Intravenous immunoglobulins for the treatment of prolonged COVID-19 in immunocompromised patients: a brief report
Abstract
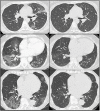
Primary humoral deficiency and secondary B-cell depletion may lead to prolonged Sars-Cov-2 infection due to a decreased viral clearance. Prolonged infection is mainly driven by the lack of anti-Sars-Cov-2 immunoglobulin (IVIg) especially in patients with no vaccine response. Anti-spike immunoglobulin can be provided by infusion of convalescent patients' plasma: recent studies highlighted that commercial immunoglobulin show high titers of neutralizing IgG. We conducted a single center retrospective cohort. We included 9 patients (6 males, median age 74 years old): one patient with X-linked agammaglobulinemia and 8 patients treated with rituximab (2 granulomatosis with polyangiitis, 1 neuromyelitis optica, 4 low grade B-cell lymphoma and 1 EBV post-transplant lymphoproliferative disorder). Mean serum globulin was 4 ± 1.6 g/L. 7/8 had received at least 3 doses of mRNA anti-Sars-Cov-2 vaccine (median 4) with no response (anti-Spike IgG 0 for 6 patients). In this specific population requiring oxygen therapy but no intensive care support, the administration of IVIg was well tolerated and provided a swift improvement of clinical status, a significant decrease of inflammation associated to the an improvement of radiological patterns. Our results suggest that immunoglobulin could be used as a salvage therapy as an alternative to convalescent plasma but highly stringent patient selection is required due to the worldwide shortage of IVIg.
Keywords: B-cell depletion; SARS-CoV-2; immunodeficiency; intravenous immunoglobulin; rituximab.
Copyright © 2024 Billi, Cholley, Grobost, Clément, Rieu, Le Guenno and Lobbes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Intravenous immunoglobulin therapy for COVID-19 in immunocompromised patients: A retrospective cohort study.Int J Infect Dis. 2024 Jul;144:107046. doi: 10.1016/j.ijid.2024.107046. Epub 2024 Apr 12. Int J Infect Dis. 2024. PMID: 38615825
-
Case Report: Stepwise Anti-Inflammatory and Anti-SARS-CoV-2 Effects Following Convalescent Plasma Therapy With Full Clinical Recovery.Front Immunol. 2021 Apr 21;12:613502. doi: 10.3389/fimmu.2021.613502. eCollection 2021. Front Immunol. 2021. PMID: 33968017 Free PMC article.
-
SARS-CoV-2 Neutralization in Convalescent Plasma and Commercial Lots of Plasma-Derived Immunoglobulin.BioDrugs. 2022 Jan;36(1):41-53. doi: 10.1007/s40259-021-00511-9. Epub 2021 Nov 29. BioDrugs. 2022. PMID: 34843105 Free PMC article.
-
COVID-19 pneumonia in a patient with granulomatosis with polyangiitis on rituximab: case-based review.Rheumatol Int. 2021 Aug;41(8):1509-1514. doi: 10.1007/s00296-021-04905-4. Epub 2021 Jun 6. Rheumatol Int. 2021. PMID: 34091704 Free PMC article. Review.
-
Persistent COVID-19: A Case Report of an Immunocompromised Patient and a Literature Review.Acta Haematol. 2024;147(5):571-575. doi: 10.1159/000537793. Epub 2024 Feb 15. Acta Haematol. 2024. PMID: 38359806 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous