Evaluation of the Efficacy and Safety of Intravenous Immunoglobulin (IVIG) in Moderate-to-Severe Hospitalized COVID-19 Patients: A Randomized, Open-Label Parallel-Group Study
- PMID: 38808260
- PMCID: PMC11132825
- DOI: 10.1155/2024/7209380
Evaluation of the Efficacy and Safety of Intravenous Immunoglobulin (IVIG) in Moderate-to-Severe Hospitalized COVID-19 Patients: A Randomized, Open-Label Parallel-Group Study
Abstract
Purpose: Since February 2020, the world has been overwhelmed by the SARS-CoV-2 outbreak, and several patients suffered interstitial pneumonia and respiratory failure requiring mechanical ventilation, threatening the capability of healthcare systems to handle this amount of critical cases. Intravenous immunoglobulins (IVIG) possess potential immunomodulatory properties beneficial for COVID-19 patients, yet evidence supporting IVIG as adjunctive therapy remains sparse. This study evaluated the outcomes of adjunctive IVIG with the standard of care (SoC) in moderate-to-severe COVID-19 patients.
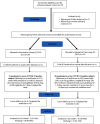
Methods: This randomized study included 59 moderate-to-severe COVID-19 patients with known comorbidities. One arm (n = 33) received high-dose IVIG (400 mg/kg/day) within 48 hours for five days alongside SoC, while the other arm (n = 26) received SoC, comprising steroids, enoxaparin, and remdesivir. The primary endpoint was clinical improvement, as measured by the National Early Warning Score 2 (NEWS2) and discharged/death proportions. Secondary outcomes included IVIG safety, hospitalization duration, changes in oxygen saturation, inflammatory markers, IgG titer, CTSS (CT severity score), and radiological findings.
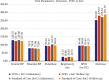
Results: There was an improvement in the NEWS2 at the end of treatment in the IVIG arm (5.67 vs. 5.96). A significant absolute effect improvement (Day 1 vs. Day 9) was seen in serum LDH, D-dimer, hs-CRP, IL-6, CTSS, procalcitonin, respiratory rate, and chest radiographic findings. SARS-CoV-2 IgG titer increased significantly in the IVIG arm. There was a statistically significant reduction in mortality in the IVIG group (5 vs. 10).
Conclusion: IVIG was a safe and effective adjunctive therapy to SoC treatment in moderate-to-severe COVID-19 patients needing ventilatory support. Furthermore, studies are required to validate our findings. This trial is registered with CTRI/2021/05/033622.
Copyright © 2024 Sachin Gautam et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Randomised controlled trial comparing efficacy and safety of high versus low Low-Molecular Weight Heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol.Trials. 2020 Jun 26;21(1):574. doi: 10.1186/s13063-020-04475-z. Trials. 2020. PMID: 32586394 Free PMC article.
-
A Phase 3 Open-label, Randomized, Controlled Study to Evaluate the Efficacy and Safety of Intravenously Administered Ravulizumab Compared with Best Supportive Care in Patients with COVID-19 Severe Pneumonia, Acute Lung Injury, or Acute Respiratory Distress Syndrome: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jul 13;21(1):639. doi: 10.1186/s13063-020-04548-z. Trials. 2020. PMID: 32660611 Free PMC article.
-
Janus kinase inhibitors for the treatment of COVID-19.Cochrane Database Syst Rev. 2022 Jun 13;6(6):CD015209. doi: 10.1002/14651858.CD015209. Cochrane Database Syst Rev. 2022. PMID: 35695334 Free PMC article. Review.
-
Intravenous immunoglobulins (IVIG) in severe/critical COVID-19 adult patients.Biomed Pharmacother. 2023 Jul;163:114851. doi: 10.1016/j.biopha.2023.114851. Epub 2023 May 5. Biomed Pharmacother. 2023. PMID: 37167723 Free PMC article. Review.
Cited by
-
Older patients affected by COVID-19: investigating the existence of biological phenotypes.BMC Geriatr. 2024 Nov 7;24(1):923. doi: 10.1186/s12877-024-05473-5. BMC Geriatr. 2024. PMID: 39511501 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous