Dexamethasone and Insulin Modulate Alanine Aminotransferase (ALT) Activity and Alanine Oxidation in C2C12 Cells in a Dose-Dependent Manner
- PMID: 38817503
- PMCID: PMC11137606
- DOI: 10.7759/cureus.59331
Dexamethasone and Insulin Modulate Alanine Aminotransferase (ALT) Activity and Alanine Oxidation in C2C12 Cells in a Dose-Dependent Manner
Abstract
Background: The muscle cells myocytes are differentiated for the purpose of contraction function, which plays a major role in body metabolism and energy haemostasis, through different metabolic pathways, such as glucose and protein metabolic pathways. Alanine aminotransferase (ALT) plays a crucial role by reversibly catalysing transamination between alanine and a-ketoglutarate to form pyruvate and glutamate and by mediating the conversion of these four major intermediate metabolites. ALT plays important roles for energy homeostasis during fasting and prolonged exercise anaerobically, when muscle protein must first be broken down into its constituent amino acids.
Methods: Mouse skeletal myoblast cell line C2C12 was cultured in Dulbecco's modified eagle medium (DMEM) growth medium, supplied with 2% horse serum supplemented with 1 uM insulin, 2 mM glutamine and penicillin and streptomycin antibiotics for seven days. The differentiation medium is refreshed every 24 hours. Then, C2C12 cells were treated with insulin and dexamethasone to examine their effects on myocytes' ALT activity.
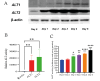
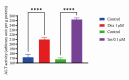
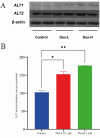
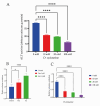
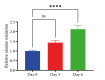
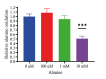
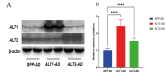
Results: In our study, we found an impact on ALT activity under different influences, including C2C12 differentiation, dexamethasone and insulin treatments, which shed light on the dynamic interplay between ALT activity, alanine metabolism, and cellular states, like differentiation and stress responses.
Conclusion: The study provides valuable insights into the dynamic regulation of ALT activity and alanine metabolism in C2C12 cells across differentiation and drug treatments. Further research is encouraged to explore the underlying mechanisms and their implications for muscle function, differentiation and potential therapeutic interventions in metabolic disorders.
Keywords: alt; c2c12; dexamethasone; expression; insulin; myocytes.
Copyright © 2024, Woraikat et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Dexamethasone effects on creatine kinase activity and insulin-like growth factor receptors in cultured muscle cells.J Cell Physiol. 1989 Jul;140(1):8-17. doi: 10.1002/jcp.1041400103. J Cell Physiol. 1989. PMID: 2544617
-
Diabetes and branched-chain amino acids: What is the link?J Diabetes. 2018 May;10(5):350-352. doi: 10.1111/1753-0407.12645. Epub 2018 Feb 13. J Diabetes. 2018. PMID: 29369529
-
PGC-1α regulates alanine metabolism in muscle cells.PLoS One. 2018 Jan 9;13(1):e0190904. doi: 10.1371/journal.pone.0190904. eCollection 2018. PLoS One. 2018. PMID: 29315328 Free PMC article.
-
Enzymes involved in l-lactate metabolism in humans.Mitochondrion. 2013 Nov;13(6):615-29. doi: 10.1016/j.mito.2013.08.011. Epub 2013 Sep 9. Mitochondrion. 2013. PMID: 24029012 Review.
-
C2C12 cell model: its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage.J Pharm Pharmacol. 2020 Dec;72(12):1667-1693. doi: 10.1111/jphp.13359. Epub 2020 Aug 18. J Pharm Pharmacol. 2020. PMID: 32812252 Review.
References
-
- C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Burattini S, Ferri P, Battistelli M, et al. Eur J Histochem. 2004;48:223–233. - PubMed
-
- Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle. Bowtell JL, Marwood S, Bruce M, Constantin-Teodosiu D, Greenhaff PL. Sports Med. 2007;37:1071–1088. - PubMed
-
- Protein metabolism and physical training: any need for amino acid supplementation? Poortmans JR, Carpentier A. Nutrire. 2016;41:21.
Grants and funding
LinkOut - more resources
Full Text Sources
