Versatile function of NF-ĸB in inflammation and cancer
- PMID: 39014491
- PMCID: PMC11251119
- DOI: 10.1186/s40164-024-00529-z
Versatile function of NF-ĸB in inflammation and cancer
Abstract
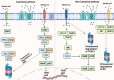
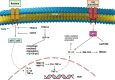
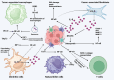
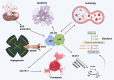
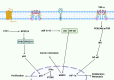
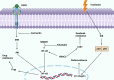
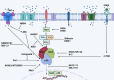
Nuclear factor-kappaB (NF-ĸB) plays a crucial role in both innate and adaptive immune systems, significantly influencing various physiological processes such as cell proliferation, migration, differentiation, survival, and stemness. The function of NF-ĸB in cancer progression and response to chemotherapy has gained increasing attention. This review highlights the role of NF-ĸB in inflammation control, biological mechanisms, and therapeutic implications in cancer treatment. NF-ĸB is instrumental in altering the release of inflammatory factors such as TNF-α, IL-6, and IL-1β, which are key in the regulation of carcinogenesis. Specifically, in conditions including colitis, NF-ĸB upregulation can intensify inflammation, potentially leading to the development of colorectal cancer. Its pivotal role extends to regulating the tumor microenvironment, impacting components such as macrophages, fibroblasts, T cells, and natural killer cells. This regulation influences tumorigenesis and can dampen anti-tumor immune responses. Additionally, NF-ĸB modulates cell death mechanisms, notably by inhibiting apoptosis and ferroptosis. It also has a dual role in stimulating or suppressing autophagy in various cancers. Beyond these functions, NF-ĸB plays a role in controlling cancer stem cells, fostering angiogenesis, increasing metastatic potential through EMT induction, and reducing tumor cell sensitivity to chemotherapy and radiotherapy. Given its oncogenic capabilities, research has focused on natural products and small molecule compounds that can suppress NF-ĸB, offering promising avenues for cancer therapy.
Keywords: Cancer therapy; Inflammation; NF-ĸB; Small molecule inhibitors; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Atrial natriuretic peptide down-regulates LPS/ATP-mediated IL-1β release by inhibiting NF-kB, NLRP3 inflammasome and caspase-1 activation in THP-1 cells.Immunol Res. 2016 Feb;64(1):303-12. doi: 10.1007/s12026-015-8751-0. Immunol Res. 2016. PMID: 26616294
-
Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth.Oncogene. 2015 Jul;34(27):3493-503. doi: 10.1038/onc.2014.286. Epub 2014 Sep 1. Oncogene. 2015. PMID: 25174402 Free PMC article.
-
Wogonoside prevents colitis-associated colorectal carcinogenesis and colon cancer progression in inflammation-related microenvironment via inhibiting NF-κB activation through PI3K/Akt pathway.Onco_target. 2016 Jun 7;7(23):34300-15. doi: 10.18632/onco_target.8815. Onco_target. 2016. PMID: 27102438 Free PMC article.
-
Multifaceted role of NF-κB in hepatocellular carcinoma therapy: Molecular landscape, therapeutic compounds and nanomaterial approaches.Environ Res. 2023 Jul 1;228:115767. doi: 10.1016/j.envres.2023.115767. Epub 2023 Mar 24. Environ Res. 2023. PMID: 36966991 Review.
-
The role of Nuclear Factor-kappa B signaling in human cervical cancer.Crit Rev Oncol Hematol. 2017 Dec;120:141-150. doi: 10.1016/j.critrevonc.2017.11.001. Epub 2017 Nov 7. Crit Rev Oncol Hematol. 2017. PMID: 29198328 Review.
Cited by
-
Divergent Processing of Cell Stress Signals as the Basis of Cancer Progression: Licensing NFκB on Chromatin.Int J Mol Sci. 2024 Aug 7;25(16):8621. doi: 10.3390/ijms25168621. Int J Mol Sci. 2024. PMID: 39201306 Free PMC article. Review.
References
-
- May MJ, Ghosh S. Rel/NF-κB and IκB proteins: an overview, seminars in cancer biology. Elsevier; 1997. pp. 63–73. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

