Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression
- PMID: 39099791
- PMCID: PMC11296317
- DOI: 10.2147/IJN.S459905
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression
Abstract
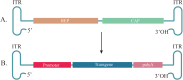
Gene therapy aims to add, replace or turn off genes to help treat disease. To date, the US Food and Drug Administration (FDA) has approved 14 gene therapy products. With the increasing interest in gene therapy, feasible gene delivery vectors are necessary for inserting new genes into cells. There are different kinds of gene delivery vectors including viral vectors like lentivirus, adenovirus, retrovirus, adeno-associated virus et al, and non-viral vectors like naked DNA, lipid vectors, polymer nanoparticles, exosomes et al, with viruses being the most commonly used. Among them, the most concerned vector is adeno-associated virus (AAV) because of its safety, natural ability to efficiently deliver gene into cells and sustained transgene expression in multiple tissues. In addition, the AAV genome can be engineered to generate recombinant AAV (rAAV) containing transgene sequences of interest and has been proven to be a safe gene vector. Recently, rAAV vectors have been approved for the treatment of various rare diseases. Despite these approvals, some major limitations of rAAV remain, namely nonspecific tissue _targeting and host immune response. Additional problems include neutralizing antibodies that block transgene delivery, a finite transgene packaging capacity, high viral titer used for per dose and high cost. To deal with these challenges, several techniques have been developed. Based on differences in engineering methods, this review proposes three strategies: gene engineering-based capsid modification (capsid modification), capsid surface tethering through chemical conjugation (surface tethering), and other formulations loaded with AAV (virus load). In addition, the major advantages and limitations encountered in rAAV engineering strategies are summarized.
Keywords: AAV engineering; capsid modification; directed evolution; machine learning; rational design; surface tethering; virus load.
© 2024 Zhou et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Delivering Transgenic DNA Exceeding the Carrying Capacity of AAV Vectors.Methods Mol Biol. 2016;1382:21-39. doi: 10.1007/978-1-4939-3271-9_2. Methods Mol Biol. 2016. PMID: 26611576 Free PMC article.
-
Mapping an Adeno-associated Virus 9-Specific Neutralizing Epitope To Develop Next-Generation Gene Delivery Vectors.J Virol. 2018 Sep 26;92(20):e01011-18. doi: 10.1128/JVI.01011-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30089698 Free PMC article.
-
Integrating adenovirus-adeno-associated virus hybrid vectors devoid of all viral genes.J Virol. 1999 Nov;73(11):9314-24. doi: 10.1128/JVI.73.11.9314-9324.1999. J Virol. 1999. PMID: 10516040 Free PMC article.
-
Tissue and cell-type-specific transduction using rAAV vectors in lung diseases.J Mol Med (Berl). 2021 Aug;99(8):1057-1071. doi: 10.1007/s00109-021-02086-y. Epub 2021 May 21. J Mol Med (Berl). 2021. PMID: 34021360 Review.
-
Adeno-Associated Virus (AAV) Gene Delivery: Dissecting Molecular Interactions upon Cell Entry.Viruses. 2021 Jul 10;13(7):1336. doi: 10.3390/v13071336. Viruses. 2021. PMID: 34372542 Free PMC article. Review.
Cited by
-
Development of an RNA virus-based episomal vector with artificial aptazyme for gene silencing.Appl Microbiol Biotechnol. 2024 Oct 18;108(1):491. doi: 10.1007/s00253-024-13327-8. Appl Microbiol Biotechnol. 2024. PMID: 39422780 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

