Establish a noninvasive model to screen metabolic dysfunction-associated steatotic liver disease in children aged 6-14 years in China and its applications in high-obesity-risk countries and regions
- PMID: 39171077
- PMCID: PMC11338159
- DOI: 10.1016/j.lanwpc.2024.101150
Establish a noninvasive model to screen metabolic dysfunction-associated steatotic liver disease in children aged 6-14 years in China and its applications in high-obesity-risk countries and regions
Abstract
Background: The prevalence of metabolic-associated steatotic liver disease (MASLD) is rising precipitously among children, particularly in regions or countries burdened with high prevalence of obesity. However, identifying those at high risk remains a significant challenge, as the majority do not exhibit distinct symptoms of MASLD. There is an urgent need for a widely accepted non-invasive predictor to facilitate early disease diagnosis and management of the disease. Our study aims to 1) evaluate and compare existing predictors of MASLD, and 2) develop a practical screening strategy for children, tailored to local prevalence of obesity.
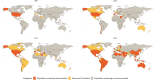
Methods: We utilized a school-based cross-sectional survey in Beijing as the training dataset to establish predictive models for screening MASLD in children. An independent school-based study in Ningbo was used to validate the models. We selected the optimal non-invasive MASLD predictor by comparing logistic regression model, random forest model, decision tree model, and support vector machine model using both the Beijing and Ningbo datasets. This was followed by serial testing using the best performance index we identified and indices from previous studies. Finally, we calculated the potential MASLD screening recommendation categories and corresponding profits based on national and subnational obesity prevalence, and applied those three categories to 200 countries according to their obesity prevalence from 1990 to 2022.
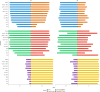
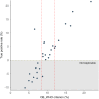
Findings: A total of 1018 children were included (NBeijing = 596, NNingbo = 422). The logistic regression model demonstrated the best performance, identifying the waist-to-height ratio (WHtR, cutoff value ≥0.48) as the optimal noninvasive index for predicting MASLD, with strong performance in both training and validation set. Additionally, the combination of WHtR and lipid accumulation product (LAP) was selected as an optimal serial test to improve the positive predictive value, with a LAP cutoff value of ≥668.22 cm × mg/dL. Based on the obesity prevalence among 30 provinces, three MASLD screening recommendations were proposed: 1) "Population-screening-recommended": For regions with an obesity prevalence ≥12.0%, where MASLD prevalence ranged from 5.0% to 21.5%; 2) "Resources-permitted": For regions with an obesity prevalence between 8.4% and 12.0%, where MASLD prevalence ranged from 2.3% to 4.4%; 3) "Population-screening-not-recommended": For regions with an obesity prevalence <8.4%, where MASLD prevalence is difficult to detect using our tool. Using our proposed cutoff for screening MASLD, the number of countries classified into the "Population-screening-recommended" and "Resources-permitted" categories increased from one and 11 in 1990 to 95 and 28 in 2022, respectively.
Interpretation: WHtR might serve as a practical and accessible index for predicting pediatric MASLD. A WHtR value ≥0.48 could facilitate early identification and management of MASLD in areas with obesity prevalence ≥12.0%. Furthermore, combining WHtR ≥0.48 with LAP ≥668.22 cm × mg/dL is recommended for individual MASLD screening. Moreover, linking these measures with population obesity prevalence not only helps estimate MASLD prevalence but also indicates potential screening profits in regions at varying levels of obesity risk.
Funding: This study was supported by grants from Capital's Funds for Health Improvement and Research (Grant No. 2022-1G-4251), National Natural Science Foundation of China (Grant No. 82273654), Major Science and Technology Projects for Health of Zhejiang Province (Grant No. WKJ-ZJ-2216), Cyrus Tang Foundation for Young Scholar 2022 (2022-B126) and Sino-German Mobility Programme (M-0015).
Keywords: Metabolic dysfunction-associated steatotic liver disease; Pediatrics; Screening recommendation categories; Waist-to-height ratio.
© 2024 The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures
Similar articles
-
Assessment of the clinical value of five noninvasive predictors of metabolic dysfunction-associated steatotic liver disease in Han Chinese adults.Eur J Gastroenterol Hepatol. 2024 Oct 1;36(10):1209-1219. doi: 10.1097/MEG.0000000000002806. Epub 2024 Jun 26. Eur J Gastroenterol Hepatol. 2024. PMID: 38973526
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Machine-Learning Application for Predicting Metabolic Dysfunction-Associated Steatotic Liver Disease Using Laboratory and Body Composition Indicators.Arch Iran Med. 2024 Oct 1;27(10):551-562. doi: 10.34172/aim.31269. Epub 2024 Oct 1. Arch Iran Med. 2024. PMID: 39492562 Free PMC article.
-
Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review From NAFLD to MAFLD and MASLD.Curr Obes Rep. 2024 Sep;13(3):510-531. doi: 10.1007/s13679-024-00574-z. Epub 2024 May 29. Curr Obes Rep. 2024. PMID: 38809396 Free PMC article. Review.
-
Lipid Screening in Childhood for Detection of Multifactorial Dyslipidemia: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-1. PMID: 27559550 Free Books & Documents. Review.
References
-
- Rinella M.E., Lazarus J.V., Ratziu V., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–1556. - PubMed
-
- Brennan P.N., Tavabie O.D., Li W., et al. Progress is impossible without change: understanding the evolving nomenclature of steatotic liver disease and its effect on hepatology practice. Lancet Gastroenterol Hepatol. 2024;9(6):577–582. - PubMed
-
- Muthiah M.D., Cheng Han N., Sanyal A.J. A clinical overview of non-alcoholic fatty liver disease: a guide to diagnosis, the clinical features, and complications-What the non-specialist needs to know. Diabetes Obes Metabol. 2022;24(Suppl 2):3–14. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

