Unveiling the Impact of BMP9 in Liver Diseases: Insights into Pathogenesis and Therapeutic Potential
- PMID: 39199400
- PMCID: PMC11353080
- DOI: 10.3390/biom14081013
Unveiling the Impact of BMP9 in Liver Diseases: Insights into Pathogenesis and Therapeutic Potential
Abstract
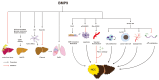
Bone morphogenetic proteins (BMPs) are a group of growth factors belonging to the transforming growth factor β(TGF-β) family. While initially recognized for their role in bone formation, BMPs have emerged as significant players in liver diseases. Among BMPs with various physiological activities, this comprehensive review aims to delve into the involvement of BMP9 specifically in liver diseases and provide insights into the complex BMP signaling pathway. Through an enhanced understanding of BMP9, we anticipate the discovery of new therapeutic options and potential strategies for managing liver diseases.
Keywords: BMP signaling; BMP9; liver cancer; liver disease; liver fibrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
New insights into BMP9 signaling in organ fibrosis.Eur J Pharmacol. 2020 Sep 5;882:173291. doi: 10.1016/j.ejphar.2020.173291. Epub 2020 Jun 20. Eur J Pharmacol. 2020. PMID: 32574673 Review.
-
Potential roles of BMP9 in liver fibrosis.Int J Mol Sci. 2014 Nov 11;15(11):20656-67. doi: 10.3390/ijms151120656. Int J Mol Sci. 2014. PMID: 25393508 Free PMC article. Review.
-
A Signaling Crosstalk between BMP9 and HGF/c-Met Regulates Mouse Adult Liver Progenitor Cell Survival.Cells. 2020 Mar 19;9(3):752. doi: 10.3390/cells9030752. Cells. 2020. PMID: 32204446 Free PMC article.
-
BMP9 is a potential therapeutic agent for use in oral and maxillofacial bone tissue engineering.Biochem Soc Trans. 2020 Jun 30;48(3):1269-1285. doi: 10.1042/BST20200376. Biochem Soc Trans. 2020. PMID: 32510140 Review.
-
BMP9-ID1 signaling promotes EpCAM-positive cancer stem cell properties in hepatocellular carcinoma.Mol Oncol. 2021 Aug;15(8):2203-2218. doi: 10.1002/1878-0261.12963. Epub 2021 May 2. Mol Oncol. 2021. PMID: 33834612 Free PMC article.
References
-
- Liu W., Deng Z., Zeng Z., Fan J., Feng Y., Wang X., Cao D., Zhang B., Yang L., Liu B., et al. Highly expressed BMP9/GDF2 in postnatal mouse liver and lungs may account for its pleiotropic effects on stem cell differentiation, angiogenesis, tumor growth and metabolism. Genes Dis. 2020;7:235–244. doi: 10.1016/j.gendis.2019.08.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

