Vaccine-induced humoral response of BNT162b2 and mRNA-1273 against BA.1, BA.5, and XBB.1.5. (sub)variants 6 months after a homologous booster: is immunogenicity equivalent?
- PMID: 39247272
- PMCID: PMC11379571
- DOI: 10.1016/j.heliyon.2024.e36116
Vaccine-induced humoral response of BNT162b2 and mRNA-1273 against BA.1, BA.5, and XBB.1.5. (sub)variants 6 months after a homologous booster: is immunogenicity equivalent?
Abstract
Introduction: Some studies suggest that the monovalent mRNA-1273 vaccine is more effective than BNT162b2 in producing higher levels of antibodies. However, limited data are available, and the methods used are not directly comparable.
Material and methods: Blood samples were obtained before the booster (third dose) and after 14, 90, and 180 days in two similar cohorts who received the original BNT162b2 or mRNA-1273 vaccine designed to _target wild type SARS-CoV-2. The aim of our study is to compare their effectiveness by assessing the levels of binding and neutralizing antibodies specifically against each of the BA.1 variant, BA.5 variant, and the XBB.1.5 subvariant.
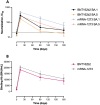
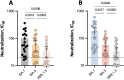
Results: Once the peak was reached after two weeks, a drastic decline in binding and neutralizing antibodies was observed up to 6 months after the homologous booster administration. The humoral response was however more sustained with the mRNA-1273 booster, with half-lives of 167, 55, and 48 days for binding, BA.1, and BA.5 neutralizing antibodies compared to 144, 30, and 29 days for the BNT162b2 booster, respectively. Compared to the BA.1 variant, the neutralizing capacity was significantly decreased at 6 months with the BA.5 variant (fold-decrease: 1.67 to 3.20) and the XBB.1.5. subvariant (fold-decrease: 2.86 to 5.48).
Conclusion: Although the decrease in the humoral response was observed with both mRNA vaccines over time, a more sustained response was observed with the mRNA-1273 vaccine. Moreover, the emergence of Omicron-based variants causes a reduced neutralizing capacity, notably with the XBB.1.5. subvariant. The administration of subsequent boosters would therefore be needed to restore a sufficiently high neutralizing response.
Keywords: BNT162b2; Humoral response; Omicron; SARS-CoV-2; Vaccine booster; mRNA-1273.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Omicron BA.1-containing mRNA-1273 boosters compared with the original COVID-19 vaccine in the UK: a randomised, observer-blind, active-controlled trial.Lancet Infect Dis. 2023 Sep;23(9):1007-1019. doi: 10.1016/S1473-3099(23)00295-5. Epub 2023 Jun 19. Lancet Infect Dis. 2023. PMID: 37348519 Clinical Trial.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Comparative immunogenicity of monovalent and bivalent adenovirus vaccines carrying spikes of early and late SARS-CoV-2 variants.Emerg Microbes Infect. 2024 Dec;13(1):2387447. doi: 10.1080/22221751.2024.2387447. Epub 2024 Aug 19. Emerg Microbes Infect. 2024. PMID: 39082740 Free PMC article.
-
Cross-neutralizing antibody against emerging Omicron subvariants of SARS-CoV-2 in infection-naïve individuals with homologous BNT162b2 or BNT162b2(WT + BA.4/5) bivalent booster vaccination.Virol J. 2024 Mar 21;21(1):70. doi: 10.1186/s12985-024-02335-9. Virol J. 2024. PMID: 38515117 Free PMC article.
-
Neutralizing Antibody Response of the Wild-Type/Omicron BA.1 Bivalent Vaccine as the Second Booster Dose against Omicron BA.2 and BA.5.Microbiol Spectr. 2023 Mar 22;11(2):e0513122. doi: 10.1128/spectrum.05131-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36946738 Free PMC article.
References
-
- Nations Unies Session extraordinaire de l'Assemblée générale consacrée à la pandémie de maladie à coronavirus (COVID-19), les 3 et 4 décembre. 2020. https://www.un.org/fr/pga/75/events 2020.
-
- European Commission First technology transfer of mRNA vaccines: working together to build new solutions. 2022. https://www.consilium.europa.eu/en/european-council/president
-
- Johns Hopkins University & Medicine . 2023. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)
-
- European Centre for Disease Prevention and Control European centre for disease prevention and control - COVID-19 vaccine tracker. 2023. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19
LinkOut - more resources
Full Text Sources
Miscellaneous

