α-Synuclein strain propagation is independent of cellular prion protein expression in a transgenic synucleinopathy mouse model
- PMID: 39264912
- PMCID: PMC11392418
- DOI: 10.1371/journal.ppat.1012517
α-Synuclein strain propagation is independent of cellular prion protein expression in a transgenic synucleinopathy mouse model
Abstract
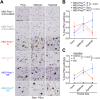
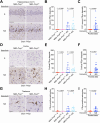
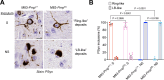
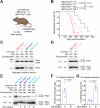
The cellular prion protein, PrPC, has been postulated to function as a receptor for α-synuclein, potentially facilitating cell-to-cell spreading and/or toxicity of α-synuclein aggregates in neurodegenerative disorders such as Parkinson's disease. Previously, we generated the "Salt (S)" and "No Salt (NS)" strains of α-synuclein aggregates that cause distinct pathological phenotypes in M83 transgenic mice overexpressing A53T-mutant human α-synuclein. To test the hypothesis that PrPC facilitates the propagation of α-synuclein aggregates, we produced M83 mice that either express or do not express PrPC. Following intracerebral inoculation with the S or NS strain, the absence of PrPC in M83 mice did not prevent disease development and had minimal influence on α-synuclein strain-specified attributes such as the extent of cerebral α-synuclein deposition, selective _targeting of specific brain regions and cell types, the morphology of induced α-synuclein deposits, and the structural fingerprints of protease-resistant α-synuclein aggregates. Likewise, there were no appreciable differences in disease manifestation between PrPC-expressing and PrPC-lacking M83 mice following intraperitoneal inoculation of the S strain. Interestingly, intraperitoneal inoculation with the NS strain resulted in two distinct disease phenotypes, indicative of α-synuclein strain evolution, but this was also independent of PrPC expression. Overall, these results suggest that PrPC plays at most a minor role in the propagation, neuroinvasion, and evolution of α-synuclein strains in mice that express A53T-mutant human α-synuclein. Thus, other putative receptors or cell-to-cell propagation mechanisms may have a larger effect on the spread of α-synuclein aggregates during disease.
Copyright: © 2024 So et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Protease-Sensitive and -Resistant Forms of Human and Murine Alpha-Synucleins in Distinct Brain Regions of Transgenic Mice (M83) Expressing the Human Mutated A53T Protein.Biomolecules. 2023 Dec 13;13(12):1788. doi: 10.3390/biom13121788. Biomolecules. 2023. PMID: 38136658 Free PMC article.
-
Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils.Acta Neuropathol Commun. 2019 May 20;7(1):80. doi: 10.1186/s40478-019-0733-3. Acta Neuropathol Commun. 2019. PMID: 31109378 Free PMC article.
-
Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological α-synuclein by enhanced ELISA.Acta Neuropathol Commun. 2014 Mar 13;2:29. doi: 10.1186/2051-5960-2-29. Acta Neuropathol Commun. 2014. PMID: 24624994 Free PMC article.
-
α-Synuclein Strains: Does Amyloid Conformation Explain the Heterogeneity of Synucleinopathies?Biomolecules. 2021 Jun 23;11(7):931. doi: 10.3390/biom11070931. Biomolecules. 2021. PMID: 34201558 Free PMC article. Review.
-
α-Synuclein Conformational Strains as Drivers of Phenotypic Heterogeneity in Neurodegenerative Diseases.J Mol Biol. 2023 Jun 15;435(12):168011. doi: 10.1016/j.jmb.2023.168011. Epub 2023 Feb 13. J Mol Biol. 2023. PMID: 36792008 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

