Safety and Tolerability of Microbial Inulinase Supplementation in Healthy Adults: A Randomized, Placebo-Controlled Trial
- PMID: 39318719
- PMCID: PMC11419904
- DOI: 10.1016/j.gastha.2024.05.013
Safety and Tolerability of Microbial Inulinase Supplementation in Healthy Adults: A Randomized, Placebo-Controlled Trial
Abstract
Background and aims: Dietary fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) contribute to gastrointestinal (GI) symptoms in individuals with FODMAP sensitivity and irritable bowel syndrome. Oral enzyme supplementation is a strategy to reduce dietary FODMAP exposure and limit FODMAP-associated GI distress. This clinical trial investigated the safety of dietary supplementation with a food-grade, microbial inulinase known to hydrolyze fructan-type or inulin-type FODMAPs and related fructo-oligosaccharides in vitro.
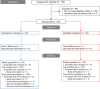
Methods: A randomized, double-blind, placebo-controlled, parallel design trial was conducted in 60 healthy adult participants of both sexes. Following a 2-week run-in placebo phase, participants were randomized to consume inulinase or placebo capsules twice daily with meals for 4 weeks. The total daily dose of inulinase was 2000 inulinase activity units. Safety measures included blood clinical chemistry, hematology, lipid profile, high-sensitivity C-reactive protein, insulin, lactate, and uric acid. GI symptoms were recorded weekly using the 15-item Gastrointestinal Symptom Rating Scale.
Results: Fifty-eight participants completed the study. There were no clinically meaningful between-group differences in blood biomarkers. During the 4-week intervention period, 5 (16.7%) of 30 participants reported 5 adverse events in the inulinase group, and 8 (26.7%) of 30 participants reported 13 adverse events in the placebo group. No statistically significant between-group differences were observed in the change from baseline to 1, 2, 3, or 4 weeks of supplementation with respect to the 15-item Gastrointestinal Symptom Rating Scale overall or domain scores.
Conclusion: Microbial inulinase supplementation demonstrated a favorable safety profile in healthy adults. Further investigation in a dose-ranging study in individuals with dietary FODMAP, fructan, or inulin sensitivity or irritable bowel syndrome is warranted. ClinicalTrials.gov: NCT05744700.
Keywords: Dietary Fiber; Enzyme; FODMAP; Food Intolerance; Food Sensitivity; Fructanase.
© 2024 The Authors.
Figures
Similar articles
-
Microbial inulinase promotes fructan hydrolysis under simulated gastric conditions.Front Nutr. 2023 May 23;10:1129329. doi: 10.3389/fnut.2023.1129329. eCollection 2023. Front Nutr. 2023. PMID: 37305092 Free PMC article.
-
Gastrointestinal tolerance of low FODMAP oral nutrition supplements in healthy human subjects: a randomized controlled trial.Nutr J. 2017 May 25;16(1):35. doi: 10.1186/s12937-017-0256-3. Nutr J. 2017. PMID: 28545589 Free PMC article. Clinical Trial.
-
A diet low in FODMAPs reduces symptoms of irritable bowel syndrome.Gastroenterology. 2014 Jan;146(1):67-75.e5. doi: 10.1053/j.gastro.2013.09.046. Epub 2013 Sep 25. Gastroenterology. 2014. PMID: 24076059 Clinical Trial.
-
Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: probiotics, prebiotics and the low FODMAP diet.Proc Nutr Soc. 2016 Aug;75(3):306-18. doi: 10.1017/S0029665116000021. Epub 2016 Feb 24. Proc Nutr Soc. 2016. PMID: 26908093 Review.
-
Dietary interventions for recurrent abdominal pain in childhood.Cochrane Database Syst Rev. 2017 Mar 23;3(3):CD010972. doi: 10.1002/14651858.CD010972.pub2. Cochrane Database Syst Rev. 2017. PMID: 28334433 Free PMC article. Review.
References
-
- Patel P.K., Tanpowpong P., Sriaroon P., et al. Nonallergic diseases associated with foods. J Allergy Clin Immunol Pract. 2024;12:607–619. - PubMed
-
- Lomer M.C. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41:262–275. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Research Materials