USP18 Is Associated with PD-L1 Antitumor Immunity and Improved Prognosis in Colorectal Cancer
- PMID: 39334957
- PMCID: PMC11430364
- DOI: 10.3390/biom14091191
USP18 Is Associated with PD-L1 Antitumor Immunity and Improved Prognosis in Colorectal Cancer
Abstract
Background: Compared with conventional chemotherapy and _targeted therapy, immunotherapy has improved the treatment outlook for a variety of solid tumors, including lung cancer, colorectal cancer (CRC), and melanoma. However, it is effective only in certain patients, necessitating the search for alternative strategies to _targeted immunotherapy. The deubiquitinating enzyme USP18 is known to play an important role in various aspects of the immune response, but its role in tumor immunity in CRC remains unclear.
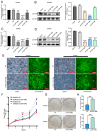
Methods: In this study, multiple online datasets were used to systematically analyze the expression, prognosis, and immunomodulatory role of USP18 in CRC. The effect of USP18 on CRC was assessed via shRNA-mediated knockdown of USP18 expression in combination with CCK-8 and colony formation assays. Finally, molecular docking analysis of USP18/ISG15 and programmed death-ligand 1 (PD-L1) was performed via HDOCK, and an ELISA was used to verify the potential of USP18 to regulate PD-L1.
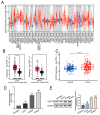
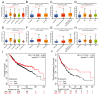
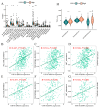
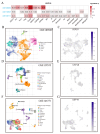
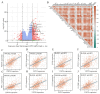
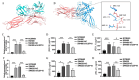
Results: Our study revealed that USP18 expression was significantly elevated in CRC patients and closely related to clinicopathological characteristics. The experimental data indicated that silencing USP18 significantly promoted the proliferation and population-dependent growth of CRC cells. In addition, high USP18 expression was positively correlated with the CRC survival rate and closely associated with tumor-infiltrating CD8+ T cells and natural killer (NK) cells. Interestingly, USP18 was correlated with the expression of various chemokines and immune checkpoint genes. The results of molecular docking simulations suggest that USP18 may act as a novel regulator of PD-L1 and that its deficiency may potentiate the antitumor immune response to PD-L1 blockade immunotherapy in CRC.
Conclusions: In summary, USP18 shows great promise for research and clinical application as a potential _target for CRC immunotherapy.
Keywords: PD-L1; USP18; anticancer immunity; colorectal cancer; immune checkpoint genes; tumor immune microenvironment.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
CMTM6 and PD-L1 coexpression is associated with an active immune microenvironment and a favorable prognosis in colorectal cancer.J Immunother Cancer. 2021 Feb;9(2):e001638. doi: 10.1136/jitc-2020-001638. J Immunother Cancer. 2021. PMID: 33579737 Free PMC article.
-
Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer.J Immunother Cancer. 2021 Jan;9(1):e001895. doi: 10.1136/jitc-2020-001895. J Immunother Cancer. 2021. PMID: 33462141 Free PMC article.
-
Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer.Mol Cancer. 2016 Aug 24;15(1):55. doi: 10.1186/s12943-016-0539-x. Mol Cancer. 2016. PMID: 27552968 Free PMC article.
-
PD-1/PD-L1-dependent immune response in colorectal cancer.J Cell Physiol. 2020 Jul;235(7-8):5461-5475. doi: 10.1002/jcp.29494. Epub 2020 Jan 21. J Cell Physiol. 2020. PMID: 31960962 Review.
-
Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer With Microsatellite Instability? Part II-The Challenge of Programmed Death Ligand-1 Testing and Its Role in Microsatellite Instability-High Colorectal Cancer.Arch Pathol Lab Med. 2018 Jan;142(1):26-34. doi: 10.5858/arpa.2017-0041-RA. Epub 2017 Nov 9. Arch Pathol Lab Med. 2018. PMID: 29120224 Review.
References
-
- GBD 2019 Colorectal Cancer Collaborators Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:627–647. doi: 10.1016/S2468-1253(22)00044-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

