Extracellular bimolecular fluorescence complementation for investigating membrane protein dimerization: a proof of concept using class B GPCRs
- PMID: 39361899
- PMCID: PMC11499381
- DOI: 10.1042/BSR20240449
Extracellular bimolecular fluorescence complementation for investigating membrane protein dimerization: a proof of concept using class B GPCRs
Abstract
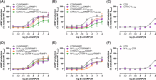
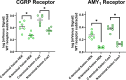
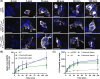
Bimolecular fluorescence complementation (BiFC) methodology uses split fluorescent proteins to detect interactions between proteins in living cells. To date, BiFC has been used to investigate receptor dimerization by splitting the fluorescent protein between the intracellular portions of different receptor components. We reasoned that attaching these split proteins to the extracellular N-terminus instead may improve the flexibility of this methodology and reduce the likelihood of impaired intracellular signal transduction. As a proof-of-concept, we used receptors for calcitonin gene-related peptide, which comprise heterodimers of either the calcitonin or calcitonin receptor-like receptor in complex with an accessory protein (receptor activity-modifying protein 1). We created fusion constructs in which split mVenus fragments were attached to either the C-termini or N-termini of receptor subunits. The resulting constructs were transfected into Cos7 and HEK293S cells, where we measured cAMP production in response to ligand stimulation, cell surface expression of receptor complexes, and BiFC fluorescence. Additionally, we investigated ligand-dependent internalization in HEK293S cells. We found N-terminal fusions were better tolerated with regards to cAMP signaling and receptor internalization. N-terminal fusions also allowed reconstitution of functional fluorescent mVenus proteins; however, fluorescence yields were lower than with C-terminal fusion. Our results suggest that BiFC methodologies can be applied to the receptor N-terminus, thereby increasing the flexibility of this approach, and enabling further insights into receptor dimerization.
Keywords: Bimolecular Fluorescence Complementation; Calcitonin gene-related peptide; calcitonin receptor; dimerization; g protein-coupled receptors; receptor activity-modifying protein.
© 2024 The Author(s).
Conflict of interest statement
D.L.H. has received research support from AbbVie and Pfizer, and has acted as an advisor, speaker, or consultant for AbbVie, Amgen, Sosei-Heptares, Teva, and Eli Lilly. C.S.W. has received research support from AbbVie. The authors declare that there are no competing interests associated with the manuscript.
Pdf file detailing the DNA and amino acid sequences of constructs and oligonucleotides used in this paper, and Supplementary Figures including immunofluorescent images and signaling assays.
Calcitonin receptor: P30988. Calcitonin receptor-like receptor: Q16602. Receptor activity-modifying protein 1: O60894
Figures




Similar articles
-
Receptor activity-modifying protein-dependent effects of mutations in the calcitonin receptor-like receptor: implications for adrenomedullin and calcitonin gene-related peptide pharmacology.Br J Pharmacol. 2014 Feb;171(3):772-88. doi: 10.1111/bph.12508. Br J Pharmacol. 2014. PMID: 24199627 Free PMC article.
-
Molecular basis of association of receptor activity-modifying protein 3 with the family B G protein-coupled secretin receptor.Biochemistry. 2009 Dec 15;48(49):11773-85. doi: 10.1021/bi901326k. Biochemistry. 2009. PMID: 19886671 Free PMC article.
-
Receptor activity-modifying protein dependent and independent activation mechanisms in the coupling of calcitonin gene-related peptide and adrenomedullin receptors to Gs.Biochem Pharmacol. 2017 Oct 15;142:96-110. doi: 10.1016/j.bcp.2017.07.005. Epub 2017 Jul 11. Biochem Pharmacol. 2017. PMID: 28705698 Free PMC article.
-
Bimolecular fluorescence complementation: lighting up seven transmembrane domain receptor signalling networks.Br J Pharmacol. 2010 Feb;159(4):738-50. doi: 10.1111/j.1476-5381.2009.00480.x. Epub 2009 Dec 10. Br J Pharmacol. 2010. PMID: 20015298 Free PMC article. Review.
-
RAMPs as allosteric modulators of the calcitonin and calcitonin-like class B G protein-coupled receptors.Adv Pharmacol. 2020;88:115-141. doi: 10.1016/bs.apha.2020.01.001. Epub 2020 Jan 27. Adv Pharmacol. 2020. PMID: 32416865 Free PMC article. Review.
References
-
- Hu C.D., Grinberg A.V. and Kerppola T.K. (2006) Visualization of protein interactions in living cells using bimolecular fluorescence complementation (BiFC) analysis. Curr. Protoc. Cell Biol. 29, 1–21Chapter 21:Unit 21 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

