Investigating the antimicrobial and antibiofilm properties of marine halophilic Bacillus species against ESKAPE pathogens
- PMID: 39446085
- PMCID: PMC11500616
- DOI: 10.1111/1758-2229.70027
Investigating the antimicrobial and antibiofilm properties of marine halophilic Bacillus species against ESKAPE pathogens
Abstract
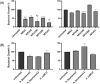
Antimicrobial resistance (AMR), known as the "silent pandemic," is exacerbated by pathogenic bacteria's ability to form biofilms. Marine compounds hold promise for novel antibacterial drug discovery. Two isolates from preliminary saltwater environment screening demonstrated antimicrobial activity and were subsequently identified as Bacillus subtilis MTUA2 and Bacillus velezensis MTUC2. Minimum inhibitory concentrations (MICs), minimum biofilm inhibition concentrations (MBICs) and minimum biofilm eradication concentrations (MBECs) required to prevent and/or disrupt bacterial growth and biofilm formation were established for MRSA, Staphylococcus aureus, Acinetobacter baumannii and Escherichia coli. The metabolic activity within biofilms was determined by the 2,3,5-triphenyltetrazolium chloride assay. Both Bacillus species exhibited unique antimicrobial effects, reducing MRSA and S. aureus planktonic cell growth by 50% and sessile cell growth for S. aureus and E. coli by 50% and 90%, respectively. No effect was observed against A. baumannii. Significant MBIC and MBEC values were achieved, with 99% inhibition and 90% reduction in MRSA and S. aureus biofilms. Additionally, 90% and 50% inhibition was observed in E. coli and A. baumannii biofilms, respectively, with a 50% reduction in E. coli biofilm. These findings suggest that the mode of action employed by B. subtilis MTUA2 and B. velezensis MTUC2 metabolites should be further characterized and could be beneficial if used independently or in combination with other treatments.
© 2024 The Author(s). Environmental Microbiology Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The antimicrobial and antibiofilm effects of gentamicin, imipenem, and fucoidan combinations against dual-species biofilms of Staphylococcus aureus and Acinetobacter baumannii isolated from diabetic foot ulcers.Ann Clin Microbiol Antimicrob. 2024 Nov 15;23(1):101. doi: 10.1186/s12941-024-00760-w. Ann Clin Microbiol Antimicrob. 2024. PMID: 39548455 Free PMC article.
-
Norfloxacin salts of carboxylic acids curtail planktonic and biofilm mode of growth in ESKAPE pathogens.J Appl Microbiol. 2018 Feb;124(2):408-422. doi: 10.1111/jam.13651. J Appl Microbiol. 2018. PMID: 29178633
-
Bacillus subtilis-derived peptides disrupt quorum sensing and biofilm assembly in multidrug-resistant Staphylococcus aureus.mSystems. 2024 Aug 20;9(8):e0071224. doi: 10.1128/msystems.00712-24. Epub 2024 Jul 11. mSystems. 2024. PMID: 38990088 Free PMC article.
-
Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections.BMC Microbiol. 2016 Jun 23;16(1):119. doi: 10.1186/s12866-016-0737-0. BMC Microbiol. 2016. PMID: 27339028 Free PMC article.
-
Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens.BMC Microbiol. 2014 Aug 14;14:197. doi: 10.1186/1471-2180-14-197. BMC Microbiol. 2014. PMID: 25124936 Free PMC article.
References
-
- Al‐Dulaimi, M. , Algburi, A. , Abdelhameed, A. , Mazanko, M.S. , Rudoy, D.V. , Ermakov, A.M. et al. (2021) Antimicrobial and anti‐biofilm activity of Polymyxin E alone and in combination with probiotic strains of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B‐1895 against clinical isolates of selected Acinetobacter spp.: a preliminary study. Pathogens, 10, 1574. Available from: 10.3390/pathogens10121574 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

