French Version of the User Mobile Application Rating Scale: Adaptation and Validation Study
- PMID: 39447142
- PMCID: PMC11527390
- DOI: 10.2196/63776
French Version of the User Mobile Application Rating Scale: Adaptation and Validation Study
Abstract
Background: Managing noncommunicable diseases effectively requires continuous coordination and monitoring, often facilitated by eHealth technologies like mobile health (mHealth) apps. The end-user version of the Mobile Application Rating Scale is a valuable tool for assessing the quality of mHealth apps from the user perspective. However, the absence of a French version restricts its use in French-speaking countries, where the evaluation and regulation of mHealth apps are still lacking, despite the increasing number of apps and their strong relevance in health care.
Objective: This study aims to translate and culturally adapt a French version of the user Mobile Application Rating Scale (uMARS-F) and to test its overall and internal reliability.
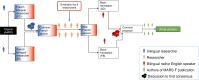
Methods: Cross-cultural adaptation and translation followed the universalist approach. The uMARS-F was evaluated as part through a cohort study using the French mHealth app "MonSherpa" (Qare). Participants were French-speaking adults with Apple or Android phones, excluding those with difficulty understanding French, prior app use, or physical limitations. They assessed the app using the uMARS-F twice (T1 and T2) 1 week apart. Scores for each section and overall were assessed for normal distribution using the Shapiro-Wilk test and presented as mean (SD), and potential floor or ceiling effects were calculated accordingly. Overall reliability was evaluated using intraclass correlation coefficients and internal reliability using Cronbach α. Concordance between the 3 subscales (objective quality, subjective quality, and perceived impact), 4 sections, and 26 items at T1 and T2 was evaluated using the paired t test (2-tailed) and Pearson correlation.
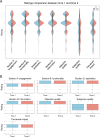
Results: In total, 167 participants assessed the app at both T1 and T2 (100% compliance). Among them, 49.7% (n=83) were female, and 50.3% (n=84) were male, with a mean age of 43 (SD 16) years. The uMARS-F intraclass correlation coefficients were excellent for objective quality (0.959), excellent for subjective quality (0.993), and moderate for perceived impact (0.624). Cronbach α was good for objective quality (0.881), acceptable for subjective quality (0.701), and excellent for perceived impact (0.936). The paired t tests (2-tailed) demonstrated similar scores between the 2 assessments (P>.05), and the Pearson correlation coefficient indicated high consistency in each subscale, section, and item (r>0.76 and P<.001). The reliability and validity of the measures were similar to those found in the original English version as well as in the Spanish, Japanese, Italian, Greek, and Turkish versions that have already been translated and validated.
Conclusions: The uMARS-F is a valid tool for end users to assess the quality of mHealth apps in French-speaking countries. The uMARS-F used in combination with the French version of the Mobile Application Rating Scale could enable health care professionals and public health authorities to identify reliable, high-quality, and valid apps for patients and should be part of French health care education programs.
Keywords: Mobile Application Rating Scale, user version; eHealth; mHealth; mobile apps; mobile health; mobile health apps; quality assessment tool; uMARS.
© Ina Saliasi, Romain Lan, Maryem Rhanoui, Laurie Fraticelli, Stéphane Viennot, Delphine Tardivo, Céline Clément, Benjamin du Sartz de Vigneulles, Sandie Bernard, Adeline Darlington-Bernard, Claude Dussart, Denis Bourgeois, Florence Carrouel. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org).
Conflict of interest statement
None declared.
Figures
Similar articles
-
Development and validation of the Japanese version of the uMARS (user version of the mobile app rating system).Int J Med Inform. 2022 Sep;165:104809. doi: 10.1016/j.ijmedinf.2022.104809. Epub 2022 Jun 13. Int J Med Inform. 2022. PMID: 35728358
-
Spanish adaptation and validation of the User Version of the Mobile Application Rating Scale (uMARS).J Am Med Inform Assoc. 2021 Nov 25;28(12):2681-2686. doi: 10.1093/jamia/ocab216. J Am Med Inform Assoc. 2021. PMID: 34613400 Free PMC article.
-
Promoting Health via mHealth Applications Using a French Version of the Mobile App Rating Scale: Adaptation and Validation Study.JMIR Mhealth Uhealth. 2021 Aug 31;9(8):e30480. doi: 10.2196/30480. JMIR Mhealth Uhealth. 2021. PMID: 34463623 Free PMC article.
-
Tools for Evaluating the Content, Efficacy, and Usability of Mobile Health Apps According to the Consensus-Based Standards for the Selection of Health Measurement Instruments: Systematic Review.JMIR Mhealth Uhealth. 2021 Dec 1;9(12):e15433. doi: 10.2196/15433. JMIR Mhealth Uhealth. 2021. PMID: 34855618 Free PMC article. Review.
-
Quality and Accessibility of Home Assessment mHealth Apps for Community Living: Systematic Review.JMIR Mhealth Uhealth. 2024 Mar 11;12:e52996. doi: 10.2196/52996. JMIR Mhealth Uhealth. 2024. PMID: 38466987 Free PMC article. Review.
References
-
- Noncommunicable diseases. World Health Organization. 2023. [22-06-2024]. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases URL. Accessed.
-
- GBD 2021 Diseases and Injuries Collaborators Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024 May 18;403(10440):2133–2161. doi: 10.1016/S0140-6736(24)00757-8. doi. Medline. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

