Safety, efficacy and immunogenicity of aerosolized Ad5-nCoV COVID-19 vaccine in a non-inferiority randomized controlled trial
- PMID: 39482336
- PMCID: PMC11527888
- DOI: 10.1038/s41541-024-01003-x
Safety, efficacy and immunogenicity of aerosolized Ad5-nCoV COVID-19 vaccine in a non-inferiority randomized controlled trial
Abstract
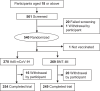
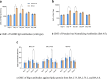
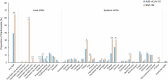
This phase 3, observer-blinded, non-inferiority randomized trial (ClinicalTrials.gov: NCT05517642), conducted from September 2022 to May 2023 at three Malaysian sites, involved 540 adults previously vaccinated with three COVID-19 doses. Participants were randomized 1:1 to receive either one dose of inhaled Recombinant COVID-19 Vaccine (Ad5-nCoV-IH) or intramuscular tozinameran (BNT-IM). The study assessed safety, vaccine efficacy (VE) and immunogenicity against SARS-CoV-2 variants. The primary outcome was the non-inferiority of anti-spike protein receptor-binding domain (S-RBD IgG) antibodies, with a 97.5% confidence interval lower limit for the geometric mean concentration (GMC) ratio >0.67. Ad5-nCoV-IH showed lower immunogenicity than BNT-IM, with a GMC ratio of 0.22 and a seroconversion rate difference of -71.91%. Adverse drug reactions (ADRs) were less frequent with Ad5-nCoV-IH (39.26%) compared to BNT-IM (64.68%). No serious vaccine-related adverse events were reported. Both vaccines had comparable efficacy against COVID-19 variants. This study was funded by Tianjin Biomedical Science and Technology Major Project.
© 2024. The Author(s).
Conflict of interest statement
T.Z., J.G., R. W., S.A, H.H., X.Y.Z, L.W., M.Y., Y.L. and V.K.C. reported being employed by CanSino Biologics Inc. which manufacture the study vaccine, during the conduct of the study and outside the submitted work. J.G. and T.Z. reported holding stock in CanSino Biologics Inc. T.O.L. reported serving as a paid senior scientific advisor to CanSino Biologics Inc. S.Ng., D.N., S.B., R.Y., N.Z., S.S.C., A.A.A., C.C.K. and, L.H.P. reported receiving research grants via Clinical Research Malaysia (CRM) through a contract with CanSino Biologics Inc. during the conduct of the study and outside the submitted work. R.R. and X.W. received financial support from CanSino Biologics Inc. for contracted work outside the submitted work. X.X.X and J.S.T. received financial support from CanSino Biologics Inc. for contracted work outside the submitted work. G.Y.C., A.M.N, K.Y.C, Y.L.L, W.H.W.M, M.R.M.D., W.M.R.W.A.K. and M.H.T. reported receiving research grants from Clinical Research Malaysia (CRM) through a contract with CanSino Biologics Inc. No other disclosures were reported.
Figures
Similar articles
-
Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial.Lancet Infect Dis. 2021 Dec;21(12):1654-1664. doi: 10.1016/S1473-3099(21)00396-0. Epub 2021 Jul 26. Lancet Infect Dis. 2021. PMID: 34324836 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of heterologous boosting with orally administered aerosolized bivalent adenovirus type-5 vectored COVID-19 vaccine and B.1.1.529 variant adenovirus type-5 vectored COVID-19 vaccine in adults 18 years and older: a randomized, double blinded, parallel controlled trial.Emerg Microbes Infect. 2024 Dec;13(1):2281355. doi: 10.1080/22221751.2023.2281355. Epub 2023 Dec 30. Emerg Microbes Infect. 2024. PMID: 37933089 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a protein subunit COVID-19 vaccine (ZF2001) in healthy children and adolescents aged 3-17 years in China: a randomised, double-blind, placebo-controlled, phase 1 trial and an open-label, non-randomised, non-inferiority, phase 2 trial.Lancet Child Adolesc Health. 2023 Apr;7(4):269-279. doi: 10.1016/S2352-4642(22)00376-5. Epub 2023 Feb 17. Lancet Child Adolesc Health. 2023. PMID: 36803632 Free PMC article. Clinical Trial.
-
A China-developed adenovirus vector-based COVID-19 vaccine: review of the development and application of Ad5-nCov.Expert Rev Vaccines. 2023 Jan-Dec;22(1):704-713. doi: 10.1080/14760584.2023.2242528. Expert Rev Vaccines. 2023. PMID: 37501516 Review.
-
Safety, immunogenicity, and protective effective of inhaled COVID-19 vaccines: A systematic review and meta-analysis.J Med Virol. 2024 Apr;96(4):e29625. doi: 10.1002/jmv.29625. J Med Virol. 2024. PMID: 38650361 Review.
References
-
- Halperin, S. A. et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet399, 237–248 (2022). - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

