The double-edged role and therapeutic potential of TREM2 in atherosclerosis
- PMID: 39497214
- PMCID: PMC11533605
- DOI: 10.1186/s40364-024-00675-w
The double-edged role and therapeutic potential of TREM2 in atherosclerosis
Abstract
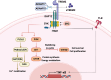
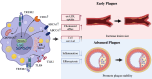
Atherosclerosis is a chronic lipid-driven inflammatory disease characterized by infiltration of large numbers of macrophages. The progression of the disease is closely related to the status of macrophages in atherosclerotic plaques. Recent advances in plaque analysis have revealed a subpopulation of macrophages that express high levels of triggering receptor expressed on myeloid cells 2 (TREM2). Although TREM2 is known to play a critical role in inflammation, lipid metabolism, and tissue repair, its role in atherosclerosis is still not fully understood. Recent studies have shown that TREM2 promotes macrophage cholesterol uptake and efflux, enhances efferocytosis function, regulates inflammation and metabolism, and promotes cell survival, all of which are significant functions in atherosclerosis. In early plaques TREM2 promotes lipid uptake and increases lesion size. In advanced plaques TREM2 promotes macrophage survival and increases plaque stability. The dualistic nature of TREM2 in atherosclerosis, where it can exert both protective effect and a side effect of increased lesion size, presents a complex but crucial area of study. Understanding these dual roles could help in the development of new therapeutic strategies to modulate TREM2 activity and utilize its atheroprotective function while mitigating its deleterious effects. In this review, we discuss the roles and mechanisms of TREM2 during different stages of atherosclerotic plaques, as well as the potential applications of TREM2 in the diagnosis and treatment of atherosclerosis.
Keywords: Atherosclerosis; Macrophage; Plaque stability; TREM2; Therapeutic strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare that they have no competing interests.
Figures
Similar articles
-
Trem2 Agonist Reprograms Foamy Macrophages to Promote Atherosclerotic Plaque Stability-Brief Report.Arterioscler Thromb Vasc Biol. 2024 Jul;44(7):1646-1657. doi: 10.1161/ATVBAHA.124.320797. Epub 2024 May 2. Arterioscler Thromb Vasc Biol. 2024. PMID: 38695172 Free PMC article.
-
TREM2 promotes cholesterol uptake and foam cell formation in atherosclerosis.Cell Mol Life Sci. 2023 May 3;80(5):137. doi: 10.1007/s00018-023-04786-9. Cell Mol Life Sci. 2023. PMID: 37133566 Free PMC article.
-
Trem2 promotes foamy macrophage lipid uptake and survival in atherosclerosis.Nat Cardiovasc Res. 2023 Nov;2(11):1015-1031. doi: 10.1038/s44161-023-00354-3. Epub 2023 Oct 30. Nat Cardiovasc Res. 2023. PMID: 38646596 Free PMC article.
-
New Classification of Macrophages in Plaques: a Revolution.Curr Atheroscler Rep. 2020 Jun 18;22(8):31. doi: 10.1007/s11883-020-00850-y. Curr Atheroscler Rep. 2020. PMID: 32556603 Review.
-
Characteristics of plaque lipid-associated macrophages and their possible roles in the pathogenesis of atherosclerosis.Curr Opin Lipidol. 2022 Oct 1;33(5):283-288. doi: 10.1097/MOL.0000000000000842. Epub 2022 Aug 3. Curr Opin Lipidol. 2022. PMID: 35942822 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

