HIF-1α knockdown attenuates phenotypic transformation and oxidative stress induced by high salt in human aortic vascular smooth muscle cells
- PMID: 39543255
- PMCID: PMC11564746
- DOI: 10.1038/s41598-024-79892-0
HIF-1α knockdown attenuates phenotypic transformation and oxidative stress induced by high salt in human aortic vascular smooth muscle cells
Abstract
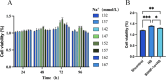
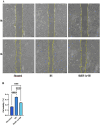
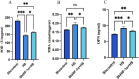
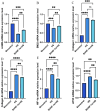
Increased dietary salt intake is a well-established risk factor for hypertension and related cardiovascular diseases, involving complex vascular remodeling processes. However, the specific role of hypoxia-inducible factor-1α (HIF-1α) in vascular pathophysiology under high-salt conditions remains poorly understood. This study investigates the role of HIF-1α in high-salt-induced vascular remodeling using human aortic vascular smooth muscle cells (HA-VSMCs) cultured in vitro. HA-VSMCs were divided into three groups: high-salt with HIF-1α knockdown (shHIF-1α + HS), negative control (shcontrol), and high-salt (HS). Cell viability, migration, gene expression, and protein levels were evaluated. High-salt conditions significantly increased mRNA expression of α-smooth muscle actin (α-SMA), smooth muscle protein 22 (SM22), angiotensin II type 1 receptor (AT1R), collagen I, and collagen III (p < 0.0001). HIF-1α knockdown partially attenuated these increases, particularly for α-SMA, SM22, and AT1R (p < 0.01). At the protein level, high-salt exposure markedly elevated expression of collagen III, HIF-1α, osteopontin (OPN), and angiotensin II (Ang II) (p < 0.0001). HIF-1α knockdown significantly reduced the high-salt-induced increases in collagen III and HIF-1α protein levels (p < 0.001) but had a limited effect on OPN and Ang II upregulation. Interestingly, SM22 protein expression was significantly decreased under high-salt conditions (p < 0.0001), an effect partially reversed by HIF-1α knockdown (p < 0.0001). These findings demonstrate that high-salt conditions induce complex changes in gene and protein expression in HA-VSMCs, with HIF-1α playing a crucial role in mediating many of these alterations. The study highlights the differential effects of HIF-1α on various markers of vascular remodeling and suggests that HIF-1α may be a potential therapeutic _target for mitigating salt-induced vascular pathology. Further research is warranted to elucidate the mechanisms underlying the HIF-1α-dependent and -independent effects observed in this study.
Keywords: Angiotensin II; Human aortic vascular smooth muscle cell; Hypoxia-inducible factor-1α; Salt; Vascular remodeling.
© 2024. The Author(s).
Conflict of interest statement
Figures

Similar articles
-
Hypoxia inducible factor 1α in vascular smooth muscle cells promotes angiotensin II-induced vascular remodeling via activation of CCL7-mediated macrophage recruitment.Cell Death Dis. 2019 Jul 18;10(8):544. doi: 10.1038/s41419-019-1757-0. Cell Death Dis. 2019. PMID: 31320613 Free PMC article.
-
[Mechanism of salidroside in inhibiting proliferation, migration and promoting phenotypic switching of arterial smooth muscle cells].Zhongguo Zhong Yao Za Zhi. 2024 Jun;49(12):3356-3364. doi: 10.19540/j.cnki.cjcmm.20240325.201. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39041099 Chinese.
-
Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells.J Cell Biochem. 2005 Dec 1;96(5):971-85. doi: 10.1002/jcb.20559. J Cell Biochem. 2005. PMID: 16149050
-
Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via HIF-1alpha dependent pathway.BMC Cell Biol. 2010 Aug 20;11:66. doi: 10.1186/1471-2121-11-66. BMC Cell Biol. 2010. PMID: 20727156 Free PMC article.
-
Hypoxia-Inducible Factor-1α in Smooth Muscle Cells Protects Against Aortic Aneurysms-Brief Report.Arterioscler Thromb Vasc Biol. 2016 Nov;36(11):2158-2162. doi: 10.1161/ATVBAHA.116.307784. Epub 2016 Aug 25. Arterioscler Thromb Vasc Biol. 2016. PMID: 27562915
References
-
- Wei, C. et al. Resolvin D1 attenuates Ang II-induced hypertension in mice by inhibiting the proliferation, migration and phenotypic transformation of vascular smooth muscle cells by blocking the RhoA/mitogen-activated protein kinase pathway. J. Hypertens.42, 420–431. 10.1097/hjh.0000000000003610 (2024). - PMC - PubMed
-
- Hansen-Smith, F. M., Morris, L. W., Greene, A. S. & Lombard, J. H. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ. Res.79, 324–330. 10.1161/01.res.79.2.324 (1996). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

