Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis
- PMID: 9632810
- PMCID: PMC109010
- DOI: 10.1128/MCB.18.7.4262
Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis
Abstract
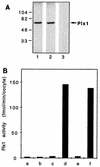
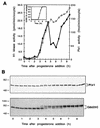
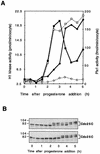
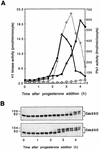
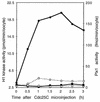
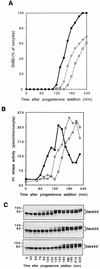
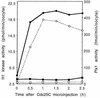
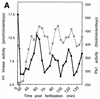
Entry into mitosis depends upon activation of the dual-specificity phosphatase Cdc25C, which dephosphorylates and activates the cyclin B-Cdc2 complex. Previous work has shown that the Xenopus polo-like kinase Plx1 can phosphorylate and activate Cdc25C in vitro. In the work presented here, we demonstrate that Plx1 is activated in vivo during oocyte maturation with the same kinetics as Cdc25C. Microinjection of wild-type Plx1 into Xenopus oocytes accelerated the rate of activation of Cdc25C and cyclin B-Cdc2. Conversely, microinjection of either an antibody against Plx1 or kinase-dead Plx1 significantly inhibited the activation of Cdc25C and cyclin B-Cdc2. This effect could be reversed by injection of active Cdc25C, indicating that Plx1 is upstream of Cdc25C. However, injection of Cdc25C, which directly activates cyclin B-Cdc2, also caused activation of Plx1, suggesting that a positive feedback loop exists in the Plx1 activation pathway. Other experiments show that injection of Plx1 antibody into early embryos, which do not require Cdc25C for the activation of cyclin B-Cdc2, resulted in an arrest of cleavage that was associated with monopolar spindles. These results demonstrate that in Xenopus laevis, Plx1 plays important roles both in the activation of Cdc25C at the initiation of mitosis and in spindle assembly at late stages of mitosis.
Figures



Similar articles
-
The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes.Mol Biol Cell. 2001 Jun;12(6):1791-9. doi: 10.1091/mbc.12.6.1791. Mol Biol Cell. 2001. PMID: 11408585 Free PMC article.
-
The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs.J Cell Sci. 1998 Jun;111 ( Pt 12):1751-7. doi: 10.1242/jcs.111.12.1751. J Cell Sci. 1998. PMID: 9601104
-
Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1.Mol Cell Biol. 1999 Dec;19(12):8625-32. doi: 10.1128/MCB.19.12.8625. Mol Cell Biol. 1999. PMID: 10567586 Free PMC article.
-
Xenopus Polo-like kinase Plx1: a multifunctional mitotic kinase.Oncogene. 2005 Jan 10;24(2):238-47. doi: 10.1038/sj.onc.1208220. Oncogene. 2005. PMID: 15640839 Review.
-
Regulation of Cdc25C activity during the meiotic G2/M transition.Cell Cycle. 2004 Jun;3(6):733-7. Epub 2004 Jun 8. Cell Cycle. 2004. PMID: 15136768 Review.
Cited by
-
A dynamical model of oocyte maturation unveils precisely orchestrated meiotic decisions.PLoS Comput Biol. 2012 Jan;8(1):e1002329. doi: 10.1371/journal.pcbi.1002329. Epub 2012 Jan 5. PLoS Comput Biol. 2012. PMID: 22238511 Free PMC article.
-
Normal cells, but not cancer cells, survive severe Plk1 depletion.Mol Cell Biol. 2006 Mar;26(6):2093-108. doi: 10.1128/MCB.26.6.2093-2108.2006. Mol Cell Biol. 2006. PMID: 16507989 Free PMC article.
-
The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe.EMBO J. 2001 Mar 15;20(6):1259-70. doi: 10.1093/emboj/20.6.1259. EMBO J. 2001. PMID: 11250892 Free PMC article.
-
Metaphase arrest with centromere separation in polo mutants of Drosophila.J Cell Biol. 2001 May 14;153(4):663-76. doi: 10.1083/jcb.153.4.663. J Cell Biol. 2001. PMID: 11352929 Free PMC article.
-
Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest.J Cell Biol. 2003 Dec 22;163(6):1231-42. doi: 10.1083/jcb.200306153. J Cell Biol. 2003. PMID: 14691134 Free PMC article.
References
-
- Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355:368–371. - PubMed
-
- Dasso M, Newport J W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. - PubMed
-
- Dunphy W G, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
