Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage
- PMID: 9696790
- PMCID: PMC109918
- DOI: 10.1128/JVI.72.9.6988-6996.1998
Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage
Abstract
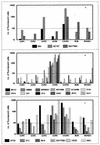
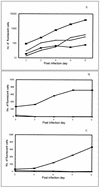
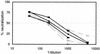
Most strains of human immunodeficiency virus type 1 (HIV-1) which have only been carried in vitro in peripheral blood mononuclear cells (primary isolates) can be neutralized by antibodies, but their sensitivity to neutralization varies considerably. To study the parameters that contribute to the differential neutralization sensitivity of primary HIV-1 isolates, we developed a neutralization assay with a panel of genetically engineered cell lines (GHOST cells) that express CD4, one of eight chemokine receptors which function as HIV-1 coreceptors, and a Tat-dependent green fluorescent protein reporter cassette which permits the evaluation and quantitation of HIV-1 infection by flow cytometry. All 21 primary isolates from several clades could grow in the various GHOST cell lines, and their use of one or more coreceptors could easily be defined by flow cytometric analysis. Ten of these primary isolates, three that were CXCR4 (X4)-tropic, three that were CCR5 (R5)-tropic, and four that were dual- or polytropic were chosen for study of their sensitivity to neutralization by human monoclonal and polyclonal antibodies. Viruses from the X4-tropic category of viruses were first tested since they have generally been considered to be particularly neutralization sensitive. It was found that the X4-tropic virus group contained both neutralization-sensitive and neutralization-resistant viruses. Similar results were obtained with R5-tropic viruses and with dual- or polytropic viruses. Within each category of viruses, neutralization sensitivity and resistance could be observed. Therefore, sensitivity to neutralization appears to be the consequence of factors that influence the antibody-virus interaction and its sequelae rather than coreceptor usage. Neutralization of various viruses by the V3-specific monoclonal antibody, 447-52D, was shown to be dependent not only on the presence of the relevant epitope but also on its presentation. An epitope within the envelope of a particular virus is not sufficient to render a virus sensitive to neutralization by an antibody that recognizes that epitope. Moreover, conformation-dependent factors may overcome the need for absolute fidelity in the match between an antibody and its core epitope, permitting sufficient affinity between the viral envelope protein and the antibody to neutralize the virus. The studies indicate that the neutralization sensitivity of HIV-1 primary isolates is a consequence of the complex interaction between virus, antibody, and _target cell.
Figures
Similar articles
-
Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains.J Virol. 2005 Jun;79(11):6957-68. doi: 10.1128/JVI.79.11.6957-6968.2005. J Virol. 2005. PMID: 15890935 Free PMC article.
-
V3 loop truncations in HIV-1 envelope impart resistance to coreceptor inhibitors and enhanced sensitivity to neutralizing antibodies.PLoS Pathog. 2007 Aug 24;3(8):e117. doi: 10.1371/journal.ppat.0030117. PLoS Pathog. 2007. PMID: 17722977 Free PMC article.
-
HIV-1 R5 Macrophage-Tropic Envelope Glycoprotein Trimers Bind CD4 with High Affinity, while the CD4 Binding Site on Non-macrophage-tropic, T-Tropic R5 Envelopes Is Occluded.J Virol. 2018 Jan 2;92(2):e00841-17. doi: 10.1128/JVI.00841-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29118121 Free PMC article.
-
Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells.Virology. 1997 Mar 17;229(2):360-9. doi: 10.1006/viro.1997.8443. Virology. 1997. PMID: 9126249
-
Chemokines and chemokine receptors in susceptibility to HIV-1 infection and progression to AIDS.Dis Markers. 2012;32(3):143-51. doi: 10.3233/DMA-2011-0874. Dis Markers. 2012. PMID: 22377730 Free PMC article. Review.
Cited by
-
Identification of HIV inhibitors guided by free energy perturbation calculations.Curr Pharm Des. 2012;18(9):1199-216. doi: 10.2174/138161212799436421. Curr Pharm Des. 2012. PMID: 22316150 Free PMC article. Review.
-
Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells.J Virol. 2007 Sep;81(17):8933-43. doi: 10.1128/JVI.00878-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567699 Free PMC article.
-
Functional chimeras of human immunodeficiency virus type 1 Gp120 and influenza A virus (H3) hemagglutinin.J Virol. 2005 May;79(10):6459-71. doi: 10.1128/JVI.79.10.6459-6471.2005. J Virol. 2005. PMID: 15858029 Free PMC article.
-
Phenotypic and genotypic characterization of human immunodeficiency virus type 1 CRF07_BC strains circulating in the Xinjiang Province of China.Retrovirology. 2009 May 14;6:45. doi: 10.1186/1742-4690-6-45. Retrovirology. 2009. PMID: 19442296 Free PMC article.
-
Immunotyping of human immunodeficiency virus type 1 (HIV): an approach to immunologic classification of HIV.J Virol. 1999 May;73(5):4042-51. doi: 10.1128/JVI.73.5.4042-4051.1999. J Virol. 1999. PMID: 10196300 Free PMC article.
References
-
- Arendrup M, Sonnerborg A, Svennerholm B, Akerblom L, Nielsen C, Clausen H, Olofsson S, Nielsen J O, Hansen J S. Neutralizing antibody response during human immunodeficiency virus type 1 infection: type and group specificity and viral escape. J Gen Virol. 1993;74:855–863. - PubMed
-
- Armstrong S J, McInerney T L, McLain L, Wahren B, Hinkula J, Levi M, Dimmock N J. Two neutralizing anti-V3 monoclonal antibodies act by affecting different functions of human immunodeficiency virus type 1. J Gen Virol. 1996;77:2931–2941. - PubMed
-
- Auewarakul P, Louisirirotchanakul S, Sutthent R, Taechowisan T, Kanoksinsombat C, Wasi C. Analysis of neutralizing and enhancing antibodies to human immunodeficiency virus type 1 primary isolates in plasma of individuals infected with env genetic subtype B and E viruses in Thailand. Viral Immunol. 1996;9:175–185. - PubMed
-
- Back N K, Smit L, Hogervorst E, van Wijk A C, Goudsmit J, Tersmette M. Development and evaluation of an HIV-1 transfection-neutralization assay. J Acquired Immune Defic Syndr. 1994;7:531–538. - PubMed
-
- Bandres J C, Wang Q F, O’Leary J, Baleaux F, Amara A, Hoxie J, Zolla-Pazner S, Gorny M K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

