The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex
- PMID: 9733861
- PMCID: PMC110166
- DOI: 10.1128/JVI.72.10.8191-8197.1998
The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex
Abstract
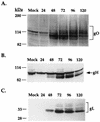
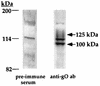
The human cytomegalovirus (HCMV) gCIII envelope complex is composed of glycoprotein H (gH; gpUL75), glycoprotein L (gL; gpUL115), and a third, 125-kDa protein not related to gH or gL (M. T. Huber and T. Compton, J. Virol. 71:5391-5398, 1997; L. Li, J. A. Nelson, and W. J. Britt, J. Virol. 71:3090-3097, 1997). Glycosidase digestion analysis demonstrated that the 125-kDa protein was a glycoprotein containing ca. 60 kDa of N-linked oligosaccharides on a peptide backbone of 65 kDa or less. Based on these biochemical characteristics, two HCMV open reading frames, UL74 and TRL/IRL12, were identified as candidate genes for the 125-kDa glycoprotein. To identify the gene encoding the 125-kDa glycoprotein, we purified the gCIII complex, separated the components by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and subjected gH and the 125-kDa glycoprotein to amino acid microsequence analysis. Microsequencing of an internal peptide derived from purified 125-kDa glycoprotein yielded the amino acid sequence LYVGPTK. A FASTA search revealed an exact match of this sequence to amino acids 188 to 195 of the predicted product of the candidate gene UL74, which we have designated glycoprotein O (gO). Anti-gO antibodies reacted in immunoblots with a protein species migrating at ca. 100 to 125 kDa in lysates of HCMV-infected cells and with 100- and 125-kDa protein species in purified virions. Anti-gO antibodies also immunoprecipitated the gCIII complex and recognized the 125-kDa glycoprotein component of the gCIII complex. Positional homologs of the UL74 gene were found in other betaherpesviruses, and comparisons of the predicted products of the UL74 homolog genes demonstrated a number of conserved biochemical features.
Figures
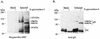

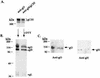

Similar articles
-
Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex.J Virol. 1997 Jul;71(7):5391-8. doi: 10.1128/JVI.71.7.5391-5398.1997. J Virol. 1997. PMID: 9188610 Free PMC article.
-
Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex.J Virol. 1997 Apr;71(4):3090-7. doi: 10.1128/JVI.71.4.3090-3097.1997. J Virol. 1997. PMID: 9060671 Free PMC article.
-
Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus.J Virol. 1999 May;73(5):3886-92. doi: 10.1128/JVI.73.5.3886-3892.1999. J Virol. 1999. PMID: 10196283 Free PMC article.
-
Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46.J Virol. 2004 May;78(9):4609-16. doi: 10.1128/jvi.78.9.4609-4616.2004. J Virol. 2004. PMID: 15078943 Free PMC article.
-
The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates.J Virol. 2002 Nov;76(21):10841-8. doi: 10.1128/jvi.76.21.10841-10848.2002. J Virol. 2002. PMID: 12368327 Free PMC article.
Cited by
-
Role of Envelope Glycoprotein Complexes in Cell-Associated Spread of Human Cytomegalovirus.Viruses. 2021 Apr 2;13(4):614. doi: 10.3390/v13040614. Viruses. 2021. PMID: 33918406 Free PMC article.
-
DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression.J Virol. 1999 Jul;73(7):5757-66. doi: 10.1128/JVI.73.7.5757-5766.1999. J Virol. 1999. PMID: 10364327 Free PMC article.
-
A Homolog Pentameric Complex Dictates Viral Epithelial Tropism, Pathogenicity and Congenital Infection Rate in Guinea Pig Cytomegalovirus.PLoS Pathog. 2016 Jul 7;12(7):e1005755. doi: 10.1371/journal.ppat.1005755. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27387220 Free PMC article.
-
Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A.J Virol. 1999 Oct;73(10):8040-52. doi: 10.1128/JVI.73.10.8040-8052.1999. J Virol. 1999. PMID: 10482553 Free PMC article.
-
Human Cytomegalovirus Cell Tropism and Host Cell Receptors.Vaccines (Basel). 2019 Jul 22;7(3):70. doi: 10.3390/vaccines7030070. Vaccines (Basel). 2019. PMID: 31336680 Free PMC article. Review.
References
-
- Barnett B C, Dolan A, Telford E A R, Davison A J, McGeoch D J. A novel herpes simplex virus gene (UL49A) encodes a putative membrane protein with counterparts in other herpesviruses. J Gen Virol. 1992;73:2167–2171. - PubMed
-
- Britt W J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984;135:369–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

