The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals
- PMID: 9891055
- PMCID: PMC116050
- DOI: 10.1128/MCB.19.2.1210
The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals
Abstract
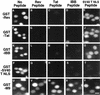
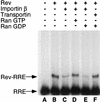
Protein nuclear import is generally mediated by basic nuclear localization signals (NLSs) that serve as _targets for the importin alpha (Imp alpha) NLS receptor. Imp alpha is in turn bound by importin beta (Imp beta), which _targets the resultant protein complex to the nucleus. Here, we report that the arginine-rich NLS sequences present in the human immunodeficiency virus type 1 regulatory proteins Tat and Rev fail to interact with Imp alpha and instead bind directly to Imp beta. Using in vitro nuclear import assays, we demonstrate that Imp alpha is entirely dispensable for Tat and Rev nuclear import. In contrast, Imp beta proved both sufficient and necessary, in that other beta-like import factors, such as transportin, were unable to support Tat or Rev nuclear import. Using in vitro competition assays, it was demonstrated that the _target sites on Imp beta for Imp alpha, Tat, and Rev binding either are identical or at least overlap. The interaction of Tat and Rev with Imp beta is also similar to Imp alpha binding in that it is inhibited by RanGTP but not RanGDP, a finding that may in part explain why the interaction of the Rev nuclear RNA export factor with _target RNA species is efficient in the cell nucleus yet is released in the cytoplasm. Together, these studies define a novel class of arginine-rich NLS sequences that are direct _targets for Imp beta and that therefore function independently of Imp alpha.
Figures


Similar articles
-
Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta.J Mol Biol. 1997 Dec 19;274(5):693-707. doi: 10.1006/jmbi.1997.1420. J Mol Biol. 1997. PMID: 9405152
-
Importin beta can mediate the nuclear import of an arginine-rich nuclear localization signal in the absence of importin alpha.Mol Cell Biol. 1999 Feb;19(2):1218-25. doi: 10.1128/MCB.19.2.1218. Mol Cell Biol. 1999. PMID: 9891056 Free PMC article.
-
Multiple importins function as nuclear transport receptors for the Rev protein of human immunodeficiency virus type 1.J Biol Chem. 2006 Jul 28;281(30):20883-20890. doi: 10.1074/jbc.M602189200. Epub 2006 May 16. J Biol Chem. 2006. PMID: 16704975
-
Structural variety of membrane permeable peptides.Curr Protein Pept Sci. 2003 Apr;4(2):87-96. doi: 10.2174/1389203033487261. Curr Protein Pept Sci. 2003. PMID: 12678848 Review.
-
The HIV-1 Tat transactivator protein: a therapeutic _target?IUBMB Life. 2003 Dec;55(12):669-80. doi: 10.1080/15216540310001643440. IUBMB Life. 2003. PMID: 14769003 Review.
Cited by
-
Bioinformatics and Functional Analysis of a New Nuclear Localization Sequence of the Influenza A Virus Nucleoprotein.Cells. 2022 Sep 22;11(19):2957. doi: 10.3390/cells11192957. Cells. 2022. PMID: 36230922 Free PMC article.
-
Off to the organelles - killing cancer cells with _targeted gold nanoparticles.Theranostics. 2015 Jan 21;5(4):357-70. doi: 10.7150/thno.10657. eCollection 2015. Theranostics. 2015. PMID: 25699096 Free PMC article. Review.
-
Functional analysis of the simian immunodeficiency virus Vpx protein: identification of packaging determinants and a novel nuclear _targeting domain.J Virol. 2001 Jan;75(1):362-74. doi: 10.1128/JVI.75.1.362-374.2001. J Virol. 2001. PMID: 11119605 Free PMC article.
-
Exchange of the basic domain of human immunodeficiency virus type 1 Rev for a polyarginine stretch expands the RNA binding specificity, and a minimal arginine cluster is required for optimal RRE RNA binding affinity, nuclear accumulation, and trans-activation.J Virol. 2001 Mar;75(6):2957-71. doi: 10.1128/JVI.75.6.2957-2971.2001. J Virol. 2001. PMID: 11222721 Free PMC article.
-
Nuclear-cytoplasmic shuttling of Chibby controls beta-catenin signaling.Mol Biol Cell. 2010 Jan 15;21(2):311-22. doi: 10.1091/mbc.e09-05-0437. Epub 2009 Nov 25. Mol Biol Cell. 2010. PMID: 19940019 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
