Maximize productivity by capturing, processing, and integrating data from multiple sources and vendors on a compliant electronic data capture (EDC) system.
Finally, a modern clinical trials platform that is both powerful and easy to use
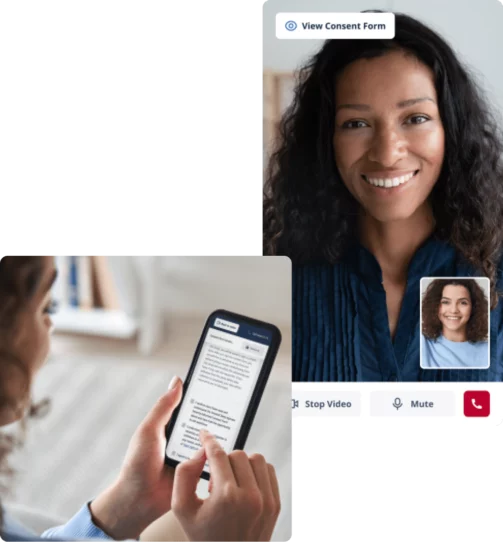
Create seamless patient experiences.
Have an easy time doing it.

Every trial is different. See how Castor can help you
-
ePRO
Say goodbye to paper surveys. Capture and manage patient data on a centralized, electronic clinical data platform.
-
eConsent
Create effortless experiences for patients with an all-in-one solution to remotely recruit, screen and enroll patients from the comfort of their home.
-
DCT
How Castor EDC, eConsent, ePRO & API can help your trials succeed in the era of COVID-19 & beyond.
-
Medical Device & Diagnostics
Manage data for your entire medical device and diagnostics development lifecycle and reduce time-to-market.
-
Biotech & Pharma
Efficiently build and deploy clinical trials that leverage data from any source on our self-service platform.
-
Contract Research Organizations
Deliver the traditional clinical trial approaches of today while offering a pathway to successfully manage hybrid and decentralized trials in the future.
-
Academic Research
Join the community of thousands of researchers across the world who are using Castor’s user-friendly and affordable software for their studies.
Why Castor?
Connect every aspect of your clinical data
- Capture and integrate data from any source
- Monitor study health and progress through real-time reporting
- Gain efficiency, visibility and data quality across the trial lifecycle
Approach data capture with confidence
- Store sensitive personal and research data securely
- Compliant with 21 CFR part 11, ICH e6 GCP, GDPR and HIPAA
- ISO27001 and ISO9001 certified
Implement and oversee DCT tech with ease
- User-friendly platform for sites and investigators
- Proactive support team to help ensure your study is successful
#0
Ranked EDC
0 K +
Happy Users
0 K +
Studies
0 %
Customer Satisfaction
0 +
Countries
0 M +
Patients
0 %
On time delivery
Over 500 trusted partners


Featured resource
Capturing data from many sources with Castor EDC
RSP Systems' GlucoBeam monitor makes it easier for patients to capture glucose readings at home. Learn how we helped them capture all their data in a single and secure system.










