Detection of Lymph Node Metastases in Human Colorectal Cancer by Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence with Spectral Unmixing
Abstract
:1. Introduction
2. Results
2.1. Separation of Overlapping Fluorescence Signals Using Spectral Unmixing
2.2. Enrolled Patients and LNs
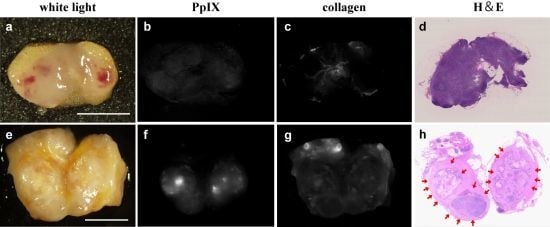
2.3. Imaging of Excised LNs in CRC Patients
2.4. Diagnostic Performance of 5-ALA-Induced PpIX by Using the Spectral Unmixing Method
3. Discussion
4. Methods
4.1. Patients
4.2. 5-ALA Administration
4.3. Tissue Processing
4.4. Acquisition of Fluorescence Images and Reference Spectra for Spectral Unmixing
4.5. Spectral Unmixing
4.6. Statistical Analysis
5. Conclusions




| Case number | Age (years) | Sex | Histological grade | Tumor location | Tumor depth (T) | Nodal status (N) | Stage | Number of examined nodes |
|---|---|---|---|---|---|---|---|---|
| 1 | 81 | Female | Diff. | A | T4a | N2b | IIIC | 3 |
| 2 | 75 | Female | Diff. | A | T3 | N1a | IIIB | 6 |
| 3 | 74 | Female | Diff. | S | T4a | N0 | IIB | 4 |
| 4 | 70 | Male | Diff. | S | T2 | N0 | I | 4 |
| 5 | 65 | Male | Diff. | A | T3 | N1b | IIIB | 4 |
| 6 | 63 | Male | Diff. | A | T3 | N0 | IIA | 3 |
| 7 | 67 | Male | Diff. | D | T4a | N1b | IIIB | 5 |
| 8 | 69 | Male | Diff. | S | T2 | N0 | I | 7 |
| 9 | 66 | Male | Diff. | R | T3 | N1a | IIIB | 5 |
| 10 | 46 | Male | Diff. | R | T3 | N2b | IIIC | 9 |
| 11 | 72 | Male | Diff. | C | T3 | N0 | IIA | 7 |
| 12 | 61 | Female | Diff. | S | T3 | N2a | IIIB | 9 |
| 13 | 83 | Female | Undiff. | S | T4a | N1b | IIIB | 12 |
| 14 | 56 | Male | Diff. | S | T3 | N2b | IIIC | 9 |
Acknowledgments
Conflicts of Interest
References
- Herszenyi, L.; Tulassay, Z. Epidemiology of gastrointestinal and liver tumors. Eur. Rev. Med. Pharmacol. Sci 2010, 14, 249–258. [Google Scholar]
- Nicholl, M.; Bilchik, A.J. Is routine use of sentinel node biopsy justified in colon cancer? Ann. Surg. Oncol 2008, 15, 1–3. [Google Scholar]
- Cohen, A.M.; Kelsen, D.; Saltz, L.; Minsky, B.D.; Nelson, H.; Farouk, R.; Gunderson, L.L.; Michelassi, F.; Arenas, R.B.; Schilsky, R.L.; et al. Adjuvant therapy for colorectal cancer. Curr. Probl. Surg 1997, 34, 601–676. [Google Scholar]
- Sigurdson, E.R. Lymph node dissection: Is it diagnostic or therapeutic? J. Clin. Oncol 2003, 21, 965–967. [Google Scholar]
- Chang, G.J.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Moyer, V.A. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J. Natl. Cancer Inst 2007, 99, 433–441. [Google Scholar]
- Baxter, N.N.; Virnig, D.J.; Rothenberger, D.A.; Morris, A.M.; Jessurun, J.; Virnig, B.A. Lymph node evaluation in colorectal cancer patients: A population-based study. J. Natl. Cancer Inst 2005, 97, 219–225. [Google Scholar]
- Iddings, D.; Ahmad, A.; Elashoff, D.; Bilchik, A. The prognostic effect of micrometastases in previously staged lymph node negative (N0) colorectal carcinoma: A meta-analysis. Ann. Surg. Oncol 2006, 13, 1386–1392. [Google Scholar]
- Le Voyer, T.E.; Sigurdson, E.R.; Hanlon, A.L.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J. Clin. Oncol 2003, 21, 2912–2919. [Google Scholar]
- Fisher, E.R.; Swamidoss, S.; Lee, C.H.; Rockette, H.; Redmond, C.; Fisher, B. Detection and significance of occult axillary node metastases in patients with invasive breast cancer. Cancer 1978, 42, 2025–2031. [Google Scholar]
- Cochran, A.J.; Wen, D.R.; Morton, D.L. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am. J. Surg. Pathol 1988, 12, 612–618. [Google Scholar]
- Trojani, M.; de Mascarel, I.; Coindre, J.M.; Bonichon, F. Micrometastases to axillary lymph nodes from invasive lobular carcinoma of breast: Detection by immunohistochemistry and prognostic significance. Br. J. Cancer 1987, 56, 838–839. [Google Scholar]
- Redding, W.H.; Coombes, R.C.; Monaghan, P.; Clink, H.M.; Imrie, S.F.; Dearnaley, D.P.; Ormerod, M.G.; Sloane, J.P.; Gazet, J.C.; Powles, T.J.; et al. Detection of micrometastases in patients with primary breast cancer. Lancet 1983, 2, 1271–1274. [Google Scholar]
- Ghossein, R.A.; Rosai, J. Polymerase chain reaction in the detection of micrometastases and circulating tumor cells. Cancer 1996, 78, 10–16. [Google Scholar]
- Wang, X.; Heller, R.; vanVoorhis, N.; Cruse, C.W.; Glass, F.; Fenske, N.; Berman, C.; Leo-Messina, J.; Rappaport, D.; Wells, K.; et al. Detection of submicroscopic lymph node metastases with polymerase chain reaction in patients with malignant melanoma. Ann. Surg 1994, 220, 768–774. [Google Scholar]
- Tamura, Y.; Kuroiwa, T.; Kajimoto, Y.; Miki, Y.; Miyatake, S.; Tsuji, M. Endoscopic identification and biopsy sampling of an intraventricular malignant glioma using a 5- aminolevulinic acid-induced protoporphyrin IX fluorescence imaging system. Technical note. J. Neurosurg 2007, 106, 507–510. [Google Scholar]
- Ray, E.R.; Chatterton, K.; Khan, M.S.; Chandra, A.; Thomas, K.; Dasgupta, P.; O’Brien, T.S. Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guerin. BJU Int 2010, 105, 789–794. [Google Scholar]
- Schmidbauer, J.; Witjes, F.; Schmeller, N.; Donat, R.; Susani, M.; Marberger, M. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J. Urol 2004, 171, 135–138. [Google Scholar]
- Regula, J.; MacRobert, A.J.; Gorchein, A.; Buonaccorsi, G.A.; Thorpe, S.M.; Spencer, G.M.; Hatfield, A.R.; Bown, S.G. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX—A pilot study. Gut 1995, 36, 67–75. [Google Scholar]
- Andersson-Engels, S.; Canti, G.; Cubeddu, R.; Eker, C.; af Klinteberg, C.; Pifferi, A.; Svanberg, K.; Svanberg, S.; Taroni, P.; Valentini, G.; et al. Preliminary evaluation of two fluorescence imaging methods for the detection and the delineation of basal cell carcinomas of the skin. Lasers Surg. Med 2000, 26, 76–82. [Google Scholar]
- Baumgartner, R.; Huber, R.M.; Schulz, H.; Stepp, H.; Rick, K.; Gamarra, F.; Leberig, A.; Roth, C. Inhalation of 5-aminolevulinic acid: A new technique for fluorescence detection of early stage lung cancer. J. Photochem. Photobiol. B 1996, 36, 169–174. [Google Scholar]
- Murayama, Y.; Harada, Y.; Imaizumi, K.; Dai, P.; Nakano, K.; Okamoto, K.; Otsuji, E.; Takamatsu, T. Precise detection of lymph node metastases in mouse rectal cancer by using 5-aminolevulinic acid. Int. J. Cancer 2009, 125, 2256–2263. [Google Scholar]
- Koizumi, N.; Harada, Y.; Murayama, Y.; Harada, K.; Beika, M.; Yamaoka, Y.; Dai, P.; Komatsu, S.; Kubota, T.; Ichikawa, D.; et al. Detection of metastatic lymph nodes using 5-aminolevulinic acid in patients with gastric cancer. Ann. Surg. Oncol 2013, 20, 3541–3548. [Google Scholar]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer _targeting and imaging with semiconductor quantum dots. Nat. Biotechnol 2004, 22, 969–976. [Google Scholar]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med 2009, 15, 104–109. [Google Scholar]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer _targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar]
- Ogawa, M.; Kosaka, N.; Choyke, P.L.; Kobayashi, H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res 2009, 69, 1268–1272. [Google Scholar]
- Murayama, Y.; Ichikawa, D.; Koizumi, N.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; Kubota, T.; Nakanishi, M.; Harada, Y.; et al. Staging fluorescence laparoscopy for gastric cancer by using 5-aminolevulinic acid. Anticancer Res 2012, 32, 5421–5427. [Google Scholar]
- Dalton, J.T.; Yates, C.R.; Yin, D.; Straughn, A.; Marcus, S.L.; Golub, A.L.; Meyer, M.C. Clinical pharmacokinetics of 5-aminolevulinic acid in healthy volunteers and patients at high risk for recurrent bladder cancer. J. Pharmacol. Exp. Ther 2002, 301, 507–512. [Google Scholar]
- Nakano, K.; Harada, Y.; Yamaoka, Y.; Miyawaki, K.; Imaizumi, K.; Takaoka, H.; Nakaoka, M.; Wakabayashi, N.; Yoshikawa, T.; Takamatsu, T. Precise analysis of the autofluorescence characteristics of rat colon under UVA and violet light excitation. Curr. Pharm. Biotechnol 2013, 14, 172–179. [Google Scholar]
- Huang, Z.; Zheng, W.; Xie, S.; Chen, R.; Zeng, H.; McLean, D.I.; Lui, H. Laser-induced autofluorescence microscopy of normal and tumor human colonic tissue. Int. J. Oncol 2004, 24, 59–63. [Google Scholar]
- Imaizumi, K.; Harada, Y.; Wakabayashi, N.; Yamaoka, Y.; Konishi, H.; Dai, P.; Tanaka, H.; Takamatsu, T. Dual-wavelength excitation of mucosal autofluorescence for precise detection of diminutive colonic adenomas. Gastrointest. Endosc 2012, 75, 110–117. [Google Scholar]
- Izuishi, K.; Tajiri, H.; Fujii, T.; Boku, N.; Ohtsu, A.; Ohnishi, T.; Ryu, M.; Kinoshita, T.; Yoshida, S. The histological basis of detection of adenoma and cancer in the colon by autofluorescence endoscopic imaging. Endoscopy 1999, 31, 511–516. [Google Scholar]
- Stummer, W.; Stepp, H.; Moller, G.; Ehrhardt, A.; Leonhard, M.; Reulen, H.J. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir 1998, 140, 995–1000. [Google Scholar]
- Fehr, M.K.; Wyss, P.; Tromberg, B.J.; Krasieva, T.; DiSaia, P.J.; Lin, F.; Tadir, Y. Selective photosensitizer localization in the human endometrium after intrauterine application of 5-aminolevulinic acid. Am. J. Obstet. Gynecol 1996, 175, 1253–1259. [Google Scholar]
- Mejia, A.; Waldmana, S.A. Previstage GCC test for staging patients with colorectal cancer. Expert Rev. Mol. Diagn 2008, 8, 571–578. [Google Scholar]
- Tsujimoto, M.; Nakabayashi, K.; Yoshidome, K.; Kaneko, T.; Iwase, T.; Akiyama, F.; Kato, Y.; Tsuda, H.; Ueda, S.; Sato, K.; et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin. Cancer Res 2007, 13, 4807–4816. [Google Scholar]
- Le Frere-Belda, M.A.; Bats, A.S.; Gillaizeau, F.; Poulet, B.; Clough, K.B.; Nos, C.; Peoc’h, M.; Seffert, P.; Bouteille, C.; Leroux, A.; et al. Diagnostic performance of one-step nucleic acid amplification for intraoperative sentinel node metastasis detection in breast cancer patients. Int. J. Cancer 2012, 130, 2377–2386. [Google Scholar]
- Berg, K.; Anholt, H.; Bech, O.; Moan, J. The influence of iron chelators on the accumulation of protoporphyrin IX in 5-aminolaevulinic acid-treated cells. Br. J. Cancer 1996, 74, 688–697. [Google Scholar]
- Hryhorenko, E.A.; Rittenhouse-Diakun, K.; Harvey, N.S.; Morgan, J.; Stewart, C.C.; Oseroff, A.R. Characterization of endogenous protoporphyrin IX induced by delta-aminolevulinic acid in resting and activated peripheral blood lymphocytes by four-color flow cytometry. Photochem. Photobiol 1998, 67, 565–572. [Google Scholar]
- Faerden, A.E.; Sjo, O.H.; Bukholm, I.R.; Andersen, S.N.; Svindland, A.; Nesbakken, A.; Bakka, A. Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Dis. Colon Rectum 2011, 54, 200–206. [Google Scholar]
- Mescoli, C.; Albertoni, L.; Pucciarelli, S.; Giacomelli, L.; Russo, V.M.; Fassan, M.; Nitti, D.; Rugge, M. Isolated tumor cells in regional lymph nodes as relapse predictors in stage I and II colorectal cancer. J. Clin. Oncol 2012, 30, 965–971. [Google Scholar]
- Rubio, C.A.; Yanagisawa, A.; Kato, Y. Histologic phenotypes of colonic carcinoma in Sweden and in Japan. Anticancer Res 1998, 18, 2649–2655. [Google Scholar]
- Garini, Y.; Young, I.T.; McNamara, G. Spectral imaging: Principles and applications. Cytometry A 2006, 69, 735–747. [Google Scholar]
- Metz, C.E.; Pan, X. Proper binormal ROC curves: Theory and maximum-likelihood estimation. J. Math. Psychol 1999, 43, 1–33. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harada, K.; Harada, Y.; Beika, M.; Koizumi, N.; Inoue, K.; Murayama, Y.; Kuriu, Y.; Nakanishi, M.; Minamikawa, T.; Yamaoka, Y.; et al. Detection of Lymph Node Metastases in Human Colorectal Cancer by Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence with Spectral Unmixing. Int. J. Mol. Sci. 2013, 14, 23140-23152. https://doi.org/10.3390/ijms141123140
Harada K, Harada Y, Beika M, Koizumi N, Inoue K, Murayama Y, Kuriu Y, Nakanishi M, Minamikawa T, Yamaoka Y, et al. Detection of Lymph Node Metastases in Human Colorectal Cancer by Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence with Spectral Unmixing. International Journal of Molecular Sciences. 2013; 14(11):23140-23152. https://doi.org/10.3390/ijms141123140
Chicago/Turabian StyleHarada, Kenichi, Yoshinori Harada, Masatomo Beika, Noriaki Koizumi, Koji Inoue, Yasutoshi Murayama, Yoshiaki Kuriu, Masayoshi Nakanishi, Takeo Minamikawa, Yoshihisa Yamaoka, and et al. 2013. "Detection of Lymph Node Metastases in Human Colorectal Cancer by Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence with Spectral Unmixing" International Journal of Molecular Sciences 14, no. 11: 23140-23152. https://doi.org/10.3390/ijms141123140
APA StyleHarada, K., Harada, Y., Beika, M., Koizumi, N., Inoue, K., Murayama, Y., Kuriu, Y., Nakanishi, M., Minamikawa, T., Yamaoka, Y., Dai, P., Yanagisawa, A., Otsuji, E., & Takamatsu, T. (2013). Detection of Lymph Node Metastases in Human Colorectal Cancer by Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence with Spectral Unmixing. International Journal of Molecular Sciences, 14(11), 23140-23152. https://doi.org/10.3390/ijms141123140