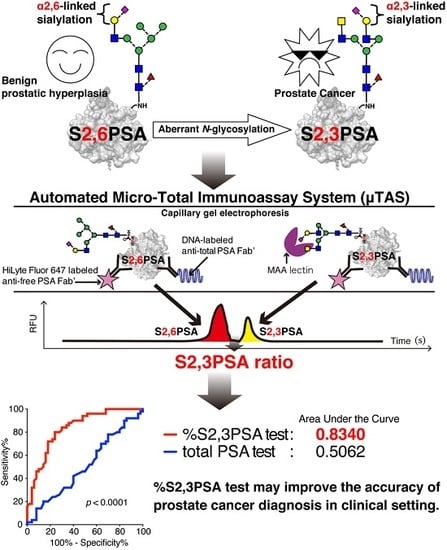
An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis
Abstract
:1. Introduction
2. Results
2.1. Preparation of FLAG-Tag-Fused Recombinat S2,3 and S2,6 PSA in Chinese Hamster Ovary (CHO) Cells
2.2. S2,6PSA and S2,3PSA Separation in a Microfluidic Channel Filled with Electrophoresis Leading Buffer Containing Maackia Amurensis Lectin
2.3. Assay Linearity and Sensitivity of %S2,3PSA Test by μTAS System
2.4. Assay Reproducibility of %S2,3PSA Test Using the μTAS System
2.5. Validation of %S2,3PSA Test
3. Discussion
4. Materials and Methods
4.1. Immunoassay Reagents
4.2. Microfluidic Electrophoresis Assay
4.3. Prostate Biopsy and Serum Samples
4.4. Forced Expression of FLAG-Tag-Fused S2,3 and S2,6PSA in Chinese Hamster Ovary (CHO)-K1 Cells
4.5. Lectin Microarray
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| μTAS | micro-total analysis system |
| MAA | maackia amurensis lectin |
| EATA | electrokinetic analyte transport assay |
| LBA | liquid-phase binding assay |
| C.V. | coefficient of variation |
| ITP | isotachophoresis |
| LIF | laser-induced fluorescence |
| LB | leading buffer |
| TB | trailing buffer |
| CE | capillary electrophoresis |
| SB | sample buffer |
| ST | stacking buffer |
| CGE | capillary gel electrophoresis |
| LOD | limit of detection |
| PSA | prostate-specific antigen |
| PCa | prostate cancer |
| BPH | benign prostatic hyperplasia |
| Pbx GG | prostate biopsy grade group |
| LacdiNAc | GalNAcβ1-4GlcNAc- |
| Gal | galactose |
| Man | mannose |
| Fuc | fucose |
| Sia | sialic acid |
| GalNAc | N-acetylgalactosamine |
| GlcNAc | N-acetylglucosamine |
References
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Carlsson, S.; Aus, G.; Bergdahl, S.; Khatami, A.; Lodding, P.; Pihl, C.G.; Stranne, J.; Holmberg, E.; Lilja, H. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010, 11, 725–732. [Google Scholar] [CrossRef]
- Loeb, S.; Catalona, W.J. Prostate-specific antigen in clinical practice. Cancer Lett. 2007, 249, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ichinose, Y.; Kubota, Y.; Imai, K.; Yamanaka, H. Clinicopathological features of prostate cancer detected by transrectal ultrasonography-guided systematic six-sextant biopsy. Int. J. Urol. 1997, 4, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ohi, M.; Yamamoto, T.; Miyamoto, S.; Kurokawa, K.; Fukabori, Y.; Suzuki, K.; Yamanaka, H. The diagnostic accuracy of the age-adjusted and prostate volume-adjusted biopsy method in males with prostate specific antigen levels of 4.1–10.0 ng/mL. Cancer 2002, 95, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.P.; Ko, Y.J.; Bubley, G.J. Biology of prostate-specific antigen. J. Clin. Oncol. 2003, 21, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Haese, A.; de la Taille, A.; Palou Redorta, J.; McNicholas, T.; Lughezzani, G.; Scattoni, V.; Bini, V.; Freschi, M.; Sussman, A.; et al. Serum isoform [−2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/mL: A multicentric European study. Eur. Urol. 2013, 63, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Sanda, M.G.; Broyles, D.L.; Shin, S.S.; Bangma, C.H.; Wei, J.T.; Partin, A.W.; Klee, G.G.; Slawin, K.M.; Marks, L.S.; et al. The prostate health index selectively identifies clinically significant prostate cancer. J. Urol. 2015, 193, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.W.; Gorman, L.; Sharifi, N.; Murphy, K.; Moore, H.; Tuzova, A.V.; Perry, A.S.; Murphy, T.B.; Lundon, D.J.; Watson, R.W. Improving multivariable prostate cancer risk assessment using the Prostate Health Index. BJU Int. 2016, 117, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Lazzeri, M.; Haese, A.; McNicholas, T.; de la Taille, A.; Buffi, N.M.; Lughezzani, G.; Gadda, G.M.; Lista, G.; Larcher, A.; et al. Clinical performance of serum isoform [−2]proPSA (p2PSA), and its derivatives %p2PSA and the Prostate Health Index, in men aged <60 years: Results from a multicentric European study. BJU Int. 2015, 115, 913–920. [Google Scholar] [PubMed]
- Punnen, S.; Pavan, N.; Parekh, D.J. Finding the Wolf in Sheep’s Clothing: The 4Kscore Is a Novel Blood Test That Can Accurately Identify the Risk of Aggressive Prostate Cancer. Rev. Urol. 2015, 17, 3–13. [Google Scholar] [PubMed]
- Vedder, M.M.; de Bekker-Grob, E.W.; Lilja, H.G.; Vickers, A.J.; van Leenders, G.J.; Steyerberg, E.W.; Roobol, M.J. The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men. Eur. Urol. 2014, 66, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996, 56, 2237–2244. [Google Scholar] [PubMed]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Taketa, K.; Nishi, S.; Fukushima, K.; Ohkura, T. Sugar chains of human cord serum α-fetoprotein: Characteristics of N-linked sugar chains of glycoproteins produced in human liver and hepatocellular carcinomas. Cancer Res. 1993, 53, 2970–2975. [Google Scholar] [PubMed]
- Amano, J.; Nishimura, R.; Mochizuki, M.; Kobata, A. Comparative study of the mucin-type sugar chains of human chorionic gonadotropin present in the urine of patients with trophoblastic diseases and healthy pregnant women. J. Biol. Chem. 1988, 263, 1157–1165. [Google Scholar] [PubMed]
- Tajiri, M.; Ohyama, C.; Wada, Y. Oligosaccharide profiles of the prostate specific antigen in free and complexed forms from the prostate cancer patient serum and in seminal plasma: A glycopeptide approach. Glycobiology 2008, 18, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, C.; Hosono, M.; Nitta, K.; Oh-eda, M.; Yoshikawa, K.; Habuchi, T.; Arai, Y.; Fukuda, M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology 2004, 14, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Ohyama, C.; Hatakeyama, S.; Narita, S.; Habuchi, T.; Koie, T.; Mori, K.; Hidari, K.I.; Yamaguchi, M.; Suzuki, T.; et al. Measurement of aberrant glycosylation of prostate specific antigen can improve specificity in early detection of prostate cancer. Biochem. Biophys. Res. Commun. 2014, 448, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.C.; Ramsey, J.M. Microchip electrophoresis with sample stacking. Electrophoresis 1995, 16, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Koutny, L.B.; Schmalzing, D.; Taylor, T.A.; Fuchs, M. Microchip electrophoretic immunoassay for serum cortisol. Anal. Chem. 1996, 68, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chiem, N.; Harrison, D.J. Microchip-based capillary electrophoresis for immunoassays: Analysis of monoclonal antibodies and theophylline. Anal. Chem. 1997, 69, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.A.; Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Wada, H.G.; Watanabe, M.; Satomura, S. Electrokinetic analyte transport assay for α-fetoprotein immunoassay integrates mixing, reaction and separation on-chip. Electrophoresis 2008, 29, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Kazakova, I.; Kawabata, T.; Spaid, M.; Chien, R.L.; Wada, H.G.; Satomura, S. Controlling data quality and reproducibility of a high-sensitivity immunoassay using isotachophoresis in a microchip. Anal. Chem. 2008, 80, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, T.; Speeckaert, M.M.; Lumen, N.; Rottey, S.; Delanghe, J.R. Glycosylation of prostate specific antigen and its potential diagnostic applications. Clin. Chim. Acta 2012, 413, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; Jones, E.E.; Powers, T.W.; Nyalwidhe, J.O. Altered glycosylation in prostate cancer. Adv. Cancer Res. 2015, 126, 345–382. [Google Scholar] [PubMed]
- Sarrats, A.; Comet, J.; Tabares, G.; Ramirez, M.; Aleixandre, R.N.; de Llorens, R.; Peracaula, R. Differential percentage of serum prostate-specific antigen subforms suggests a new way to improve prostate cancer diagnosis. Prostate 2010, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sarrats, A.; Saldova, R.; Comet, J.; O’Donoghue, N.; de Llorens, R.; Rudd, P.M.; Peracaula, R. Glycan characterization of PSA 2-DE subforms from serum and seminal plasma. Omics 2010, 14, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Watanabe, M.; Nakamura, K.; Satomura, S. Liquid-phase binding assay of α-fetoprotein using DNA-coupled antibody and capillary chip electrophoresis. Anal. Chem. 2005, 77, 5579–5582. [Google Scholar] [CrossRef] [PubMed]
- Llop, E.; Ferrer-Batalle, M.; Barrabes, S.; Guerrero, P.E.; Ramirez, M.; Saldova, R.; Rudd, P.M.; Aleixandre, R.N.; Comet, J.; de Llorens, R.; et al. Improvement of Prostate Cancer Diagnosis by Detecting PSA Glycosylation-Specific Changes. Theranostics 2016, 6, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Kagebayashi, C.; Yamaguchi, I.; Akinaga, A.; Kitano, H.; Yokoyama, K.; Satomura, M.; Kurosawa, T.; Watanabe, M.; Kawabata, T.; Chang, W.; et al. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal. Biochem. 2009, 388, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Yamada, M.; Kuno, A.; Tateno, H. Lectin microarrays: Concept, principle and applications. Chem. Soc. Rev. 2013, 42, 4443–4458. [Google Scholar] [CrossRef] [PubMed]








| Sample Number | 1.0 ng/mL Free PSA | 5.0 ng/mL Free PSA | ||
|---|---|---|---|---|
| Free PSA | %S2,3PSA | Free PSA | %S2,3PSA | |
| 1 | 1.04 | 50.5 | 5.06 | 38.1 |
| 2 | 0.99 | 50.7 | 5.08 | 38.1 |
| 3 | 1.04 | 49.4 | 5.00 | 38.3 |
| 4 | 1.05 | 50.1 | 5.07 | 37.9 |
| 5 | 1.04 | 47.7 | 5.05 | 37.7 |
| 6 | 1.03 | 50.4 | 5.24 | 37.7 |
| 7 | 1.13 | 50.3 | 5.13 | 38.3 |
| 8 | 1.10 | 46.8 | 5.01 | 37.9 |
| 9 | 1.05 | 47.1 | 5.04 | 38.1 |
| 10 | 1.01 | 48.5 | 5.21 | 37.9 |
| Ave. 1 | 1.04 | 49.1 | 5.09 | 38.0 |
| SD 2 | 0.03 | 1.51 | 0.08 | 0.21 |
| CV 3 | 2.8% | 3.1% | 1.6% | 0.6% |
| Characteristics | BPH a | PCa b | p (a vs. b) |
|---|---|---|---|
| n = 100 | 50 | 50 | |
| Age, median (range) | 66.5 (51–85) | 67 (51–86) | ns 1 |
| PSA 2, ng/mL, median (range) | 6.45 (1.9–20.4) | 6.6 (1.5–21.4) | ns 1 |
| %S2,3PSA, median (range) | 38.55 (22.9–59.1) | 45.70 (34.7–71.7) | <0.0001 |
| pbx GG 3 | n (%) | ||
| GG 1 | 15 (30%) | ||
| GG 2 | 12 (24%) | ||
| GG 3 | 8 (16%) | ||
| GG 4 | 9 (18%) | ||
| GG 5 | 6 (12%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, T.; Yoneyama, T.; Tobisawa, Y.; Hatakeyama, S.; Kurosawa, T.; Nakamura, K.; Narita, S.; Mitsuzuka, K.; Duivenvoorden, W.; Pinthus, J.H.; et al. An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2017, 18, 470. https://doi.org/10.3390/ijms18020470
Ishikawa T, Yoneyama T, Tobisawa Y, Hatakeyama S, Kurosawa T, Nakamura K, Narita S, Mitsuzuka K, Duivenvoorden W, Pinthus JH, et al. An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis. International Journal of Molecular Sciences. 2017; 18(2):470. https://doi.org/10.3390/ijms18020470
Chicago/Turabian StyleIshikawa, Tomokazu, Tohru Yoneyama, Yuki Tobisawa, Shingo Hatakeyama, Tatsuo Kurosawa, Kenji Nakamura, Shintaro Narita, Koji Mitsuzuka, Wilhelmina Duivenvoorden, Jehonathan H. Pinthus, and et al. 2017. "An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis" International Journal of Molecular Sciences 18, no. 2: 470. https://doi.org/10.3390/ijms18020470
APA StyleIshikawa, T., Yoneyama, T., Tobisawa, Y., Hatakeyama, S., Kurosawa, T., Nakamura, K., Narita, S., Mitsuzuka, K., Duivenvoorden, W., Pinthus, J. H., Hashimoto, Y., Koie, T., Habuchi, T., Arai, Y., & Ohyama, C. (2017). An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis. International Journal of Molecular Sciences, 18(2), 470. https://doi.org/10.3390/ijms18020470