Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes
Abstract
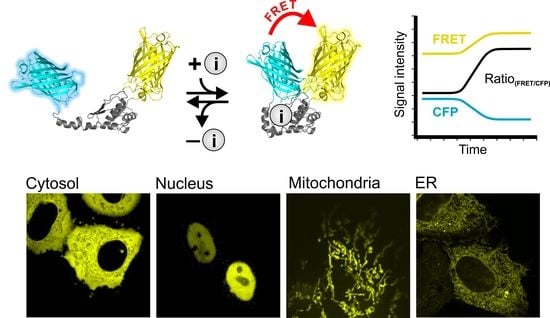
:1. Introduction
2. Genetically Encoded Fluorescent Probes for Imaging the Alkali Metal Ion K+, Highly Desired and Freshly Introduced
3. Genetically Encoded Fluorescent Probes for Alkaline Earth Metal Ions
3.1. Genetically Encoded Mg2+ Indicators, Sophisticated Tools, Rarely Applied
3.2. Genetically Encoded Ca2+ Indicators, A Huge Variety for An Ion with Versatile Roles
4. Genetically Encoded Fluorescent Probes for Transition Metal Ions
4.1. Genetically Encoded Cu+/Cu2+ Indicators, Highly Sensitive Tools for Low Concentrated Ions
4.2. Genetically Encoded Zn2+ Indicators, A Broad Palette of Applicable Probes
5. Approaches for the Design of Novel Genetically Encoded Na+, Fe2+/Fe3+ and Mn2+ Indicators
6. Concluding Remarks and Outlook
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [Ca2+] | Calcium ion concentration |
| [K+] | Potassium ion concentration |
| [K+]i | Intracellular potassium ion concentration |
| [Mg2+] | Magnesium ion concentration |
| [Mg2+]i | Intracellular magnesium ion concentration |
| [Na+] | Sodium ion concentration |
| [Na+]ex | Extracellular sodium ion concentration |
| [Zn2+] | Zinc ion concentration |
| [Zn2+]i | Intracellular zinc ion concentration |
| ACE | Angiotensin-converting enzyme |
| Ag+ | Silver ion |
| APG-1 | Asante potassium green-1 |
| BON | Bacterial OsmY and nodulation |
| Ca2+ | Calcium ion |
| CaM | Calmodulin |
| Cd2+ | Cadmium ion |
| CFP | Cyan fluorescent protein |
| Co2+ | Cobalt ion |
| Cu+/Cu2+ | Copper ions |
| ECF | Extracellular fluid |
| EMRE | Essential mitochondrial calcium uniporter regulator |
| ER | Endoplasmic reticulum |
| Fe2+/Fe3+ | Iron ions |
| FP | Fluorescent protein |
| FRET | Förster resonance energy transfer |
| GEP | Genetically encoded probe |
| GEPII | Genetically encoded potassium ion indicator |
| GFP | Green fluorescent protein |
| K+ | Potassium ion |
| Kbp | Potassium ion binding protein |
| KD | Dissociation rate constant |
| lc- | Low charge |
| LysM | Lysine motife |
| MARIO | Magnesium ratiometric indicator for optical imaging |
| MCU | Mitochondrial calcium uniporter |
| Mg2+ | Magnesium ion |
| MICU1/2 | Mitochondrial calcium uptake 1 and 2 |
| Mn2+ | Manganese ion |
| Na+ | Sodium ion |
| Pb2+ | Plumb ion |
| RFP | Red fluorescent protein |
| ROS | Reactive oxygen species |
| RpoS | RNA polymerase sigma S |
| TCR | T-cell receptor |
| TnC | Troponin C |
| YFP | Yellow fluorescent protein |
| Zn2+ | Zinc ion |
References
- Dubyak, G.R. Ion homeostasis, channels, and transporters: An update on cellular mechanisms. Adv. Physiol. Educ. 2004, 28, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Stelmashook, E.V.; Isaev, N.K.; Genrikhs, E.E.; Amelkina, G.A.; Khaspekov, L.G.; Skrebitsky, V.G.; Illarioshkin, S.N. Role of zinc and copper ions in the pathogenetic mechanisms of Alzheimer’s and Parkinson’s diseases. Biochem. Mosc. 2014, 79, 391–396. [Google Scholar] [CrossRef]
- Jansson, B. Potassium, sodium, and cancer: A review. J. Env. Pathol. Toxicol. Oncol. 1996, 15, 65–73. [Google Scholar]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J.; et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolobkova, Y.A.; Vigont, V.A.; Shalygin, A.V.; Kaznacheyeva, E.V. Huntington’s Disease: Calcium Dyshomeostasis and Pathology Models. ACTA Nat. 2017, 9, 34–46. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Schumacker, P.T.; Guzman, J.D.; Ilijic, E.; Yang, B.; Zampese, E. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1013–1019. [Google Scholar] [CrossRef]
- Fuentealba, I.C.; Aburto, E.M. Animal models of copper-associated liver disease. Comp. Hepatol. 2003, 2, 5. [Google Scholar] [CrossRef]
- Blaustein, M.P. Intracellular calcium as a second messenger. In Calcium in Biological Systems; Rubin, R.P., Weiss, G.B., Putney, J.W., Eds.; Springer: Boston, MA, USA, 1985; pp. 23–33. ISBN 978-1-4612-9453-5. [Google Scholar]
- Page, M.J.; Di Cera, E. Role of Na+ and K+ in Enzyme Function. Physiol. Rev. 2006, 86, 1049–1092. [Google Scholar] [CrossRef] [PubMed]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Gohara, D.W.; Di Cera, E. Molecular Mechanisms of Enzyme Activation by Monovalent Cations. J. Biol. Chem. 2016, 291, 20840–20848. [Google Scholar] [CrossRef] [Green Version]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [Green Version]
- Terry, J. The major electrolytes: Sodium, potassium, and chloride. J. Intraven. Nurs. 1994, 17, 240–247. [Google Scholar]
- Díaz-Soto, G.; Rocher, A.; García-Rodríguez, C.; Núñez, L.; Villalobos, C. The Calcium-Sensing Receptor in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 327, 321–369. [Google Scholar]
- Bouschet, T.; Henley, J.M. Calcium as an extracellular signalling molecule: Perspectives on the Calcium Sensing Receptor in the brain. Comptes Rendus Biol. 2005, 328, 691–700. [Google Scholar] [CrossRef]
- Harjes, D.I.; Dubach, J.M.; Rosenzweig, A.; Das, S.; Clark, H.A. Ion-Selective Optodes Measure Extracellular Potassium Flux in Excitable Cells. Macromol. Rapid Commun. 2009, 31, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Gurusamy, D.; Clever, D.; Eil, R.; Restifo, N.P. Novel “Elements” of Immune Suppression within the Tumor Microenvironment. Cancer Immunol. Res. 2017, 5, 426–433. [Google Scholar] [CrossRef]
- Hrabcová, D.; Pásek, M.; Šimurda, J.; Christé, G. Effect of Ion Concentration Changes in the Limited Extracellular Spaces on Sarcolemmal Ion Transport and Ca2+ Turnover in a Model of Human Ventricular Cardiomyocyte. Int. J. Mol. Sci. 2013, 14, 24271–24292. [Google Scholar] [CrossRef]
- Walz, W.; Hertz, L. Intracellular ion changes of astrocytes in response to extracellular potassium. J. Neurosci. Res. 1983, 10, 411–423. [Google Scholar] [CrossRef]
- Reinert, M.; Khaldi, A.; Zauner, A.; Doppenberg, E.; Choi, S.; Bullock, R. High level of extracellular potassium and its correlates after severe human head injury: Relationship to high intracranial pressure. J. Neurosurg. 2000, 93, 800–807. [Google Scholar] [CrossRef]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ. Arrhythm Electrophysiol. 2017, 10, e004667. [Google Scholar] [CrossRef]
- Espinel, E.; Joven, J.; Gil, I.; Suñé, P.; Renedo, B.; Fort, J.; Serón, D. Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: A randomized study. BMC Res. Notes 2013, 6, 306. [Google Scholar] [CrossRef]
- Raebel, M.A. Hyperkalemia Associated with Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: Hyperkalemia with ACEI and ARB. Cardiovasc. Therap. 2012, 30, e156–e166. [Google Scholar] [CrossRef]
- Luxardi, G.; Reid, B.; Ferreira, F.; Maillard, P.; Zhao, M. Measurement of Extracellular Ion Fluxes Using the Ion-selective Self-referencing Microelectrode Technique. J. Vis. Exp. 2015, 99, 52782. [Google Scholar] [CrossRef]
- Tian, L.; Looger, L.L. Genetically encoded fluorescent sensors for studying healthy and diseased nervous systems. Drug Discov. Today Dis. Models. 2008, 5, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909. [Google Scholar] [CrossRef]
- Imamura, H.; Huynh Nhat, K.P.; Togawa, H.; Saito, K.; Iino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. USA 2009, 106, 15651–15656. [Google Scholar] [CrossRef] [Green Version]
- Bischof, H.; Rehberg, M.; Stryeck, S.; Artinger, K.; Eroglu, E.; Waldeck-Weiermair, M.; Gottschalk, B.; Rost, R.; Deak, A.T.; Niedrist, T.; et al. Novel genetically encoded fluorescent probes enable real-time detection of potassium in vitro and in vivo. Nat. Commun 2017, 8, 1422. [Google Scholar] [CrossRef]
- Burgstaller, S.; Bischof, H.; Gensch, T.; Stryeck, S.; Gottschalk, B.; Ramadani-Muja, J.; Eroglu, E.; Rost, R.; Balfanz, S.; Baumann, A.; et al. pH-Lemon, a Fluorescent Protein-Based pH Reporter for Acidic Compartments. ACS Sens. 2019, 4, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Waldeck-Weiermair, M.; Bischof, H.; Blass, S.; Deak, A.; Klec, C.; Graier, T.; Roller, C.; Rost, R.; Eroglu, E.; Gottschalk, B.; et al. Generation of Red-Shifted Cameleons for Imaging Ca2+ Dynamics of the Endoplasmic Reticulum. Sensors 2015, 15, 13052–13068. [Google Scholar] [CrossRef]
- Whitaker, M. Genetically encoded probes for measurement of intracellular calcium. Methods Cell Biol. 2010, 99, 153–182. [Google Scholar]
- Lindenburg, L.H.; Vinkenborg, J.L.; Oortwijn, J.; Aper, S.J.A.; Merkx, M. MagFRET: The First Genetically Encoded Fluorescent Mg2+ Sensor. PLoS ONE 2013, 8, e82009. [Google Scholar] [CrossRef]
- Van Dongen, E.M.W.M.; Dekkers, L.M.; Spijker, K.; Meijer, E.W.; Klomp, L.W.J.; Merkx, M. Ratiometric Fluorescent Sensor Proteins with Subnanomolar Affinity for Zn(II) Based on Copper Chaperone Domains. J. Am. Chem. Soc. 2006, 128, 10754–10762. [Google Scholar] [CrossRef]
- Wegner, S.V.; Arslan, H.; Sunbul, M.; Yin, J.; He, C. Dynamic Copper(I) Imaging in Mammalian Cells with a Genetically Encoded Fluorescent Copper(I) Sensor. J. Am. Chem. Soc. 2010, 132, 2567–2569. [Google Scholar] [CrossRef]
- Lindenburg, L.; Merkx, M. Engineering genetically encoded FRET sensors. Sensors 2014, 14, 11691–11713. [Google Scholar] [CrossRef]
- Eroglu, E.; Gottschalk, B.; Charoensin, S.; Blass, S.; Bischof, H.; Rost, R.; Madreiter-Sokolowski, C.T.; Pelzmann, B.; Bernhart, E.; Sattler, W.; et al. Development of novel FP-based probes for live-cell imaging of nitric oxide dynamics. Nat. Comm. 2016, 7, 10623. [Google Scholar] [CrossRef]
- Yaginuma, H.; Kawai, S.; Tabata, K.V.; Tomiyama, K.; Kakizuka, A.; Komatsuzaki, T.; Noji, H.; Imamura, H. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci. Rep. 2015, 4. [Google Scholar] [CrossRef]
- Shen, Y.; Rosendale, M.; Campbell, R.E.; Perrais, D. pHuji, a pH-sensitive red fluorescent protein for imaging of exo- and endocytosis. J. Cell Biol. 2014, 207, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, L.; Matsuda, T.; Zhao, Y.; Rebane, A.; Drobizhev, M.; Chang, Y.-F.; Araki, S.; Arai, Y.; March, K.; et al. Improved orange and red Ca2± indicators and photophysical considerations for optogenetic applications. Acs Chem. Neurosci 2013, 4, 963–972. [Google Scholar] [CrossRef]
- Greenwald, E.C.; Mehta, S.; Zhang, J. Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem. Rev. 2018, 118, 11707–11794. [Google Scholar] [CrossRef] [PubMed]
- Leavesley, S.J.; Rich, T.C. Overcoming limitations of FRET measurements. Cytometry 2016, 89, 325–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An expanded palette of genetically encoded Ca2+ indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Pérez Koldenkova, V.; Nagai, T. Genetically encoded Ca2+ indicators: Properties and evaluation. Biochim. Et Biophys. ACTA 2013, 1833, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Woehler, A.; Wlodarczyk, J.; Neher, E. Signal/Noise Analysis of FRET-Based Sensors. Biophys. J. 2010, 99, 2344–2354. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.E.; Qin, Y.; Park, J.G.; McCombs, J.E. Design and application of genetically encoded biosensors. Trends Biotechnol. 2011, 29, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Waldeck-Weiermair, M.; Alam, M.R.; Khan, M.J.; Deak, A.T.; Vishnu, N.; Karsten, F.; Imamura, H.; Graier, W.F.; Malli, R. Spatiotemporal Correlations between Cytosolic and Mitochondrial Ca2+ Signals Using a Novel Red-Shifted Mitochondrial _targeted Cameleon. PLoS ONE 2012, 7, e45917. [Google Scholar] [CrossRef]
- Tivey, D.R.; Simmons, N.L.; Aiton, J.F. Role of passive potassium fluxes in cell volume regulation in cultured HeLa cells. J. Membr. Biol. 1985, 87, 93–105. [Google Scholar] [CrossRef]
- Clausen, M.J.V.; Poulsen, H. Sodium/Potassium Homeostasis in the Cell. Met. Ions Life Sci. 2013, 12, 41–67. [Google Scholar]
- Larkin, J.M.; Brown, M.S.; Goldstein, J.L.; Anderson, R.G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 1983, 33, 273–285. [Google Scholar] [CrossRef]
- Ceccarelli, B.; Fesce, R.; Grohovaz, F.; Haimann, C. The effect of potassium on exocytosis of transmitter at the frog neuromuscular junction. J. Physiol. 1988, 401, 163–183. [Google Scholar] [CrossRef]
- El Kebir, D.; József, L.; Khreiss, T.; Filep, J.G. Inhibition of K+ efflux prevents mitochondrial dysfunction, and suppresses caspase-3-, apoptosis-inducing factor-, and endonuclease G-mediated constitutive apoptosis in human neutrophils. Cell. Signal. 2006, 18, 2302–2313. [Google Scholar] [CrossRef] [PubMed]
- Kachmar, J.F.; Boyer, P.D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J. Biol. Chem. 1953, 200, 669–682. [Google Scholar] [PubMed]
- Kato, M.; Chuang, J.L.; Tso, S.-C.; Wynn, R.M.; Chuang, D.T. Crystal structure of pyruvate dehydrogenase kinase 3 bound to lipoyl domain 2 of human pyruvate dehydrogenase complex. EMBO J. 2005, 24, 1763–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, N.; Masuda, J.; Tobimatsu, T.; Toraya, T.; Suto, K.; Morimoto, Y.; Yasuoka, N. A new mode of B12 binding and the direct participation of a potassium ion in enzyme catalysis: X-ray structure of diol dehydratase. Structure 1999, 7, 997–1008. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.-I.; Dotson, G.; Turner, I.; Reiss, L.; Emptage, M. Crystal structure of substrate free form of glycerol dehydratase. J. Inorg. Biochem. 2003, 93, 84–91. [Google Scholar] [CrossRef]
- Andersson, C.E.; Mowbray, S.L. Activation of ribokinase by monovalent cations. J. Mol. Biol. 2002, 315, 409–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Dougherty, M.; Downs, D.M.; Ealick, S.E. Crystal structure of an aminoimidazole riboside kinase from Salmonella enterica: Implications for the evolution of the ribokinase superfamily. Structure 2004, 12, 1809–1821. [Google Scholar] [CrossRef]
- Jezek, P.; Mahdi, F.; Garlid, K.D. Reconstitution of the beef heart and rat liver mitochondrial K+/H+ (Na+/H+) antiporter. Quantitation of K+ transport with the novel fluorescent probe, PBFI. J. Biol. Chem. 1990, 265, 10522–10526. [Google Scholar]
- Rimmele, T.S.; Chatton, J.-Y. A Novel Optical Intracellular Imaging Approach for Potassium Dynamics in Astrocytes. PLoS ONE 2014, 9, e109243. [Google Scholar] [CrossRef]
- Kong, X.; Su, F.; Zhang, L.; Yaron, J.; Lee, F.; Shi, Z.; Tian, Y.; Meldrum, D.R. A Highly Selective Mitochondria-_targeting Fluorescent K+ Sensor. Angew. Chem. Int. Ed. 2015, 54, 12053–12057. [Google Scholar] [CrossRef]
- Ashraf, K.U.; Josts, I.; Mosbahi, K.; Kelly, S.M.; Byron, O.; Smith, B.O.; Walker, D. The Potassium Binding Protein Kbp Is a Cytoplasmic Potassium Sensor. Structure 2016, 24, 741–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.; Jung, K. Profiling Early Osmostress-Dependent Gene Expression in Escherichia coli Using DNA Macroarrays. J. Bacteriol. 2002, 184, 5502–5507. [Google Scholar] [CrossRef]
- Storvik, K.A.M.; Foster, P.L. RpoS, the Stress Response Sigma Factor, Plays a Dual Role in the Regulation of Escherichia coli’s Error-Prone DNA Polymerase IV. J. Bacteriol. 2010, 192, 3639–3644. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, S.-Y.; Rancic, V.; Aggarwal, A.; Qian, Y.; Miyashita, S.-I.; Ballanyi, K.; Campbell, R.E.; Dong, M. Genetically encoded fluorescent indicators for imaging intracellular potassium ion concentration. Commun. Biol. 2019, 2. [Google Scholar] [CrossRef]
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef]
- Thier, S.O. Potassium physiology. Am. J. Med. 1986, 80, 3–7. [Google Scholar] [CrossRef]
- Romani, A.M.P. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Vernon, W.B. The role of magnesium in nucleic-acid and protein metabolism. Magnesium 1988, 7, 234–248. [Google Scholar]
- Walker, G.M. Magnesium and cell cycle control: An update. Magnesium 1986, 5, 9–23. [Google Scholar]
- Noronha, J.L.; Matuschak, G.M. Magnesium in critical illness: Metabolism, assessment, and treatment. Intensive Care Med. 2002, 28, 667–679. [Google Scholar] [CrossRef]
- Bara, M.; Guiet-Bara, A.; Durlach, J. Regulation of sodium and potassium pathways by magnesium in cell membranes. Magnes Res. 1993, 6, 167–177. [Google Scholar]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Dugal, B.S.; Louis, B.M. Effect of magnesium ion (Mg2+) and the magnesium adenosine triphosphate ion (MgATP2-) on pigeon liver pyruvate carboxylase. Enzyme 1975, 20, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, L.; Garfinkel, D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium 1985, 4, 60–72. [Google Scholar] [PubMed]
- Stangherlin, A.; O’Neill, J.S. Signal Transduction: Magnesium Manifests as a Second Messenger. Curr. Biol. 2018, 28, R1403–R1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leehey, D.J. Magnesium Homeostasis in CKD. Adv. Chronic Kidney Dis. 2018, 25, 222–223. [Google Scholar] [CrossRef]
- Mountokalakis, T.D. Magnesium metabolism in chronic renal failure. Magnes Res. 1990, 3, 121–127. [Google Scholar] [PubMed]
- Bilbey, D.L.; Prabhakaran, V.M. Muscle cramps and magnesium deficiency: Case reports. Can. Fam. Physician 1996, 42, 1348–1351. [Google Scholar]
- Nuytten, D.; Van Hees, J.; Meulemans, A.; Carton, H. Magnesium deficiency as a cause of acute intractable seizures. J. Neurol. 1991, 238, 262–264. [Google Scholar] [CrossRef]
- Killilea, D.W.; Ames, B.N. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl. Acad. Sci. USA 2008, 105, 5768–5773. [Google Scholar] [CrossRef] [Green Version]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef]
- Galassi, A.; Cozzolino, M. Magnesium: A renewed player of vascular ageing in diabetic CKD patients? Clin. Kidney J. 2014, 7, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.; Murphy, E.; Levy, L.A.; Hall, R.D.; London, R.E. A fluorescent indicator for measuring cytosolic free magnesium. Am. J. Physiol. Cell Physiol. 1989, 256, C540–C548. [Google Scholar] [CrossRef]
- Salisbury, J.L. Centrin, centrosomes, and mitotic spindle poles. Curr. Opin. Cell Biol. 1995, 7, 39–45. [Google Scholar] [CrossRef]
- Cox, J.A.; Tirone, F.; Durussel, I.; Firanescu, C.; Blouquit, Y.; Duchambon, P.; Craescu, C.T. Calcium and Magnesium Binding to Human Centrin 3 and Interaction with _target Peptides. Biochemistry 2005, 44, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, K.; Matsuda, T.; Shindo, Y.; Imamura, H.; Tamura, S.; Imai, R.; Kawakami, S.; Nagashima, R.; Soga, T.; Noji, H.; et al. A Transient Rise in Free Mg2+ Ions Released from ATP-Mg Hydrolysis Contributes to Mitotic Chromosome Condensation. Curr. Biol. 2018, 28, 444–451. [Google Scholar] [CrossRef]
- Lunin, V.V.; Dobrovetsky, E.; Khutoreskaya, G.; Zhang, R.; Joachimiak, A.; Doyle, D.A.; Bochkarev, A.; Maguire, M.E.; Edwards, A.M.; Koth, C.M. Crystal structure of the CorA Mg2+ transporter. Nature 2006, 440, 833–837. [Google Scholar] [CrossRef]
- Smith, R.L.; Banks, J.L.; Snavely, M.D.; Maguire, M.E. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J. Biol. Chem. 1993, 268, 14071–14080. [Google Scholar] [PubMed]
- Sheu, S.S.; Sharma, V.K.; Banerjee, S.P. Measurement of cytosolic free calcium concentration in isolated rat ventricular myocytes with quin 2. Circ. Res. 1984, 55, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Ahvazi, B.; Boeshans, K.M.; Idler, W.; Baxa, U.; Steinert, P.M. Roles of Calcium Ions in the Activation and Activity of the Transglutaminase 3 Enzyme. J. Biol. Chem. 2003, 278, 23834–23841. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, H.I.; Sang, Q.-X.A. Calcium regulates tertiary structure and enzymatic activity of human endometase/matrilysin-2 and its role in promoting human breast cancer cell invasion. Biochem. J. 2007, 403, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, V.N.; Chagot, B.; Chazin, W.J. Calcium-Dependent Regulation of Ion Channels. Calcium Bind Proteins 2006, 1, 203–212. [Google Scholar] [PubMed]
- Hedrich, R.; Neher, E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 1987, 329, 833–836. [Google Scholar] [CrossRef]
- Van Haasteren, G.; Li, S.; Muda, M.; Susini, S.; Schlegel, W. Calcium Signalling and Gene Expression. J. Recept. Signal. Transduct. 1999, 19, 481–492. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Südhof, T.C. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef]
- Malaisse, W.J. Role of Calcium in the Regulation of Hormonal Secretion. Horm. Res. 1984, 20, 28–37. [Google Scholar] [CrossRef]
- Whitaker, M. Calcium at Fertilization and in Early Development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol.ol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Treiman, M. Regulation of the endoplasmic reticulum calcium storage during the unfolded protein response--significance in tissue ischemia? Trends Cardiovasc. Med. 2002, 12, 57–62. [Google Scholar] [CrossRef]
- Bustos, G.; Cruz, P.; Lovy, A.; Cárdenas, C. Endoplasmic Reticulum–Mitochondria Calcium Communication and the Regulation of Mitochondrial Metabolism in Cancer: A Novel Potential _target. Front. Oncol. 2017, 7, 199. [Google Scholar] [CrossRef]
- Prins, D.; Michalak, M. Organellar Calcium Buffers. Cold Spring Harb. Perspect. Biol. 2011, 3, a004069. [Google Scholar] [CrossRef]
- Koch, G.L. The endoplasmic reticulum and calcium storage. Bioessays 1990, 12, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Coe, H.; Michalak, M. Calcium binding chaperones of the endoplasmic reticulum. Gen. Physiol. Biophys. 2009, 28, F96–F103. [Google Scholar] [PubMed]
- Pedriali, G.; Rimessi, A.; Sbano, L.; Giorgi, C.; Wieckowski, M.R.; Previati, M.; Pinton, P. Regulation of Endoplasmic Reticulum–Mitochondria Ca2+ Transfer and Its Importance for Anti-Cancer Therapies. Front. Oncol. 2017, 7, 180. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Giorgi, C.; Baldassari, F.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; et al. Mitochondrial Ca2+ and apoptosis. Cell Calcium 2012, 52, 36–43. [Google Scholar] [CrossRef]
- Santo-Domingo, J.; Demaurex, N. The renaissance of mitochondrial pH. J. Gen. Physiol. 2012, 139, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graier, W.F.; Frieden, M.; Malli, R. Mitochondria and Ca2+ signaling: Old guests, new functions. Pflug. Arch. Eur J. Physiol. 2007, 455, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S.B.; Boyman, L.; Chikando, A.C.; Khairallah, R.J.; Lederer, W.J. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 10479–10486. [Google Scholar] [CrossRef] [Green Version]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]
- Griesbeck, O.; Baird, G.S.; Campbell, R.E.; Zacharias, D.A.; Tsien, R.Y. Reducing the Environmental Sensitivity of Yellow Fluorescent Protein: MECHANISM AND APPLICATIONS. J. Biol. Chem. 2001, 276, 29188–29194. [Google Scholar] [CrossRef] [PubMed]
- Truong, K.; Sawano, A.; Mizuno, H.; Hama, H.; Tong, K.I.; Mal, T.K.; Miyawaki, A.; Ikura, M. FRET-based in vivo Ca2+ imaging by a new calmodulin-GFP fusion molecule. Nat. Struct. Biol. 2001, 8, 1069–1073. [Google Scholar] [CrossRef]
- Nagai, T.; Yamada, S.; Tominaga, T.; Ichikawa, M.; Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10554–10559. [Google Scholar] [CrossRef]
- Palmer, A.E.; Jin, C.; Reed, J.C.; Tsien, R.Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA 2004, 101, 17404–17409. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.E.; Giacomello, M.; Kortemme, T.; Hires, S.A.; Lev-Ram, V.; Baker, D.; Tsien, R.Y. Ca2+ Indicators Based on Computationally Redesigned Calmodulin-Peptide Pairs. Chem. Biol. 2006, 13, 521–530. [Google Scholar] [CrossRef]
- Giacomello, M.; Drago, I.; Bortolozzi, M.; Scorzeto, M.; Gianelle, A.; Pizzo, P.; Pozzan, T. Ca2+ Hot Spots on the Mitochondrial Surface Are Generated by Ca2+ Mobilization from Stores, but Not by Activation of Store-Operated Ca2+ Channels. Mol. Cell 2010, 38, 280–290. [Google Scholar] [CrossRef]
- Ishii, K.; Hirose, K.; Iino, M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006, 7, 390–396. [Google Scholar] [CrossRef]
- Tay, L.H.; Dick, I.E.; Yang, W.; Mank, M.; Griesbeck, O.; Yue, D.T. Nanodomain Ca2+ of Ca2+ channels detected by a tethered genetically encoded Ca2+ sensor. Nat. Commun. 2012, 3, 778. [Google Scholar] [CrossRef]
- Heim, N.; Griesbeck, O. Genetically Encoded Indicators of Cellular Calcium Dynamics Based on Troponin C and Green Fluorescent Protein. J. Biol. Chem. 2004, 279, 14280–14286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mank, M.; Reiff, D.F.; Heim, N.; Friedrich, M.W.; Borst, A.; Griesbeck, O. A FRET-Based Calcium Biosensor with Fast Signal Kinetics and High Fluorescence Change. Biophys. J. 2006, 90, 1790–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzberg, O.; Moult, J.; James, M.N. Calcium binding to skeletal muscle troponin C and the regulation of muscle contraction. CIBA Found. Symp. 1986, 122, 120–144. [Google Scholar]
- Osibow, K.; Malli, R.; Kostner, G.M.; Graier, W.F. A new type of non-Ca2+-buffering Apo(a)-based fluorescent indicator for intraluminal Ca2+ in the endoplasmic reticulum. J. Biol. Chem. 2006, 281, 5017–5025. [Google Scholar] [CrossRef]
- Nagai, T.; Sawano, A.; Park, E.S.; Miyawaki, A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc. Natl. Acad. Sci. USA 2001, 98, 3197–3202. [Google Scholar] [CrossRef]
- Suzuki, J.; Kanemaru, K.; Iino, M. Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging. Biophys. J. 2016, 111, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Miyawaki, A.; Griesbeck, O.; Heim, R.; Tsien, R.Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. USA 1999, 96, 2135–2140. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, N.; Sarkar, B. Molecular mechanism of copper transport in Wilson disease. Environ. Health Perspect. 2002, 110, 695–698. [Google Scholar] [CrossRef]
- Tümer, Z.; Møller, L.B. Menkes disease. Eur J. Hum. Genet. 2010, 18, 511–518. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Klevay, L.M. Cardiovascular Disease from Copper Deficiency—A History. J. Nutr. 2000, 130, 489S–492S. [Google Scholar] [CrossRef]
- Waggoner, D.J.; Bartnikas, T.B.; Gitlin, J.D. The role of copper in neurodegenerative disease. Neurobiol. Dis. 1999, 6, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Inesi, G. Molecular features of copper binding proteins involved in copper homeostasis: Copper Binding Proteins. IUBMB Life 2017, 69, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.L.; Kaur, A.; Ratushny, A.V.; Cvetkovic, A.; Kumar, S.; Pan, M.; Arkin, A.P.; Aitchison, J.D.; Adams, M.W.W.; Baliga, N.S. Metallochaperones Regulate Intracellular Copper Levels. PLoS Comput. Biol. 2013, 9, e1002880. [Google Scholar] [CrossRef]
- Waldron, K.J.; Robinson, N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009, 7, 25–35. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.A.; Allard, S.; Santoro, N.; Côté, J.; Thiele, D.J. The Candida glabrata Amt1 copper-sensing transcription factor requires Swi/Snf and Gcn5 at a critical step in copper detoxification. Mol. Microbiol. 2001, 40, 1165–1174. [Google Scholar] [CrossRef]
- Wegner, S.V.; Sun, F.; Hernandez, N.; He, C. The tightly regulated copper window in yeast. Chem. Commun. 2011, 47, 2571–2573. [Google Scholar] [CrossRef]
- Gralla, E.B.; Thiele, D.J.; Silar, P.; Valentine, J.S. ACE1, a copper-dependent transcription factor, activates expression of the yeast copper, zinc superoxide dismutase gene. Proc. Natl. Acad. Sci. USA 1991, 88, 8558–8562. [Google Scholar] [CrossRef] [PubMed]
- Keller, G.; Bird, A.; Winge, D.R. Independent Metalloregulation of Ace1 and Mac1 in Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1863–1871. [Google Scholar] [CrossRef]
- Zhu, Z.; Labbé, S.; Peña, M.M.O.; Thiele, D.J. Copper Differentially Regulates the Activity and Degradation of Yeast Mac1 Transcription Factor. J. Biol. Chem. 1998, 273, 1277–1280. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Li, X.; Lv, S.-S.; He, D.-C. A novel genetically encoded fluorescent protein as a Cu(I) indicator. Dalton Trans. 2012, 41, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Jones, M.B.; Butler-Wu, S.; Sinsimer, D.; Gerads, R.; Bishai, W.R.; Peterson, S.N.; Darwin, K.H. A novel copper-responsive regulon in Mycobacterium tuberculosis: RicR regulon in Mycobacterium tuberculosis. Mol. Microbiol. 2011, 79, 133–148. [Google Scholar] [CrossRef]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef]
- Lim, Y.S.; Kim, S.B.; Kim, M.K.; Lim, Y.J. Disseminated Tuberculosis of Central Nervous System: Spinal Intramedullary and Intracranial Tuberculomas. J. Korean Neurosurg Soc. 2013, 54, 61. [Google Scholar] [CrossRef]
- Malaviya, A.N.; Kotwal, P.P. Arthritis associated with tuberculosis. Best Pr. Res. Clin. Rheumatol. 2003, 17, 319–343. [Google Scholar] [CrossRef]
- Daher, E.D.F.; Barros, E.J.G.; da Silva, G.B., Jr. Renal Tuberculosis in the Modern Era. Am. J. Trop. Med. Hyg. 2013, 88, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Bishai, W.R.; Dannenberg, A.M.; Parrish, N.; Ruiz, R.; Chen, P.; Zook, B.C.; Johnson, W.; Boles, J.W.; Pitt, M.L. Virulence of Mycobacterium tuberculosis CDC1551 and H37Rv in rabbits evaluated by Lurie’s pulmonary tubercle count method. Infect. Immun. 1999, 67, 4931–4934. [Google Scholar] [PubMed]
- Koay, M.S.; Janssen, B.M.G.; Merkx, M. Tuning the metal binding site specificity of a fluorescent sensor protein: From copper to zinc and back. Dalton Trans. 2013, 42, 3230–3232. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Coleman, J.E.; Auld, D.S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc. Natl. Acad. Sci. USA 1991, 88, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F. Non-Redox-Metal-Catalyzed Redox Reactions: Zinc Catalysts. Chem. Asian J. 2012, 7, 2502–2509. [Google Scholar] [CrossRef]
- Tóth, K. Zinc in neurotransmission. Annu. Rev. Nutr. 2011, 31, 139–153. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Borovanský, J.; Riley, P.A. Cytotoxicity of zinc in vitro. Chem. -Biol. Interact. 1989, 69, 279–291. [Google Scholar]
- Vinkenborg, J.L.; Nicolson, T.J.; Bellomo, E.A.; Koay, M.S.; Rutter, G.A.; Merkx, M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods 2009, 6, 737–740. [Google Scholar] [CrossRef]
- Hatori, Y.; Lutsenko, S. The Role of Copper Chaperone Atox1 in Coupling Redox Homeostasis to Intracellular Copper Distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef]
- Bartee, M.Y.; Lutsenko, S. Hepatic copper-transporting ATPase ATP7B: Function and inactivation at the molecular and cellular level. Biometals 2007, 20, 627–637. [Google Scholar] [CrossRef]
- Van Dongen, E.M.W.M.; Evers, T.H.; Dekkers, L.M.; Meijer, E.W.; Klomp, L.W.J.; Merkx, M. Variation of Linker Length in Ratiometric Fluorescent Sensor Proteins Allows Rational Tuning of Zn(II) Affinity in the Picomolar to Femtomolar Range. J. Am. Chem. Soc. 2007, 129, 3494–3495. [Google Scholar] [CrossRef]
- Dittmer, P.J.; Miranda, J.G.; Gorski, J.A.; Palmer, A.E. Genetically Encoded Sensors to Elucidate Spatial Distribution of Cellular Zinc. J. Biol. Chem. 2009, 284, 16289–16297. [Google Scholar] [CrossRef] [Green Version]
- Elrod-Erickson, M.; Rould, M.A.; Nekludova, L.; Pabo, C.O. Zif268 protein–DNA complex refined at 1.6å: A model system for understanding zinc finger–DNA interactions. Structure 1996, 4, 1171–1180. [Google Scholar] [CrossRef]
- Razin, S.V.; Borunova, V.V.; Maksimenko, O.G.; Kantidze, O.L. Cys2His2 zinc finger protein family: Classification, functions, and major members. Biochem. Mosc. 2012, 77, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.M.; Kowalski, K.; Liew, C.K.; Sharpe, B.K.; Fox, A.H.; Crossley, M.; MacKay, J.P. A class of zinc fingers involved in protein-protein interactions biophysical characterization of CCHC fingers from fog and U-shaped. Eur. J. Biochem. 2000, 267, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.M.T. Multiple modes of RNA recognition by zinc finger proteins. Curr. Opin. Struct. Biol. 2005, 15, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Evers, T.H.; Appelhof, M.A.M.; de Graaf-Heuvelmans, P.T.H.M.; Meijer, E.W.; Merkx, M. Ratiometric Detection of Zn(II) Using Chelating Fluorescent Protein Chimeras. J. Mol. Biol. 2007, 374, 411–425. [Google Scholar] [CrossRef]
- Hessels, A.M.; Chabosseau, P.; Bakker, M.H.; Engelen, W.; Rutter, G.A.; Taylor, K.M.; Merkx, M. eZinCh-2: A Versatile, Genetically Encoded FRET Sensor for Cytosolic and Intraorganelle Zn2+ Imaging. ACS Chem. Biol. 2015, 10, 2126–2134. [Google Scholar] [CrossRef]
- Qin, Y.; Dittmer, P.J.; Park, J.G.; Jansen, K.B.; Palmer, A.E. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl. Acad. Sci. USA 2011, 108, 7351–7356. [Google Scholar] [CrossRef]
- Miranda, J.G.; Weaver, A.L.; Qin, Y.; Park, J.G.; Stoddard, C.I.; Lin, M.Z.; Palmer, A.E. New Alternately Colored FRET Sensors for Simultaneous Monitoring of Zn2+ in Multiple Cellular Locations. PLoS ONE 2012, 7, e49371. [Google Scholar] [CrossRef]
- Berret, E.; Smith, P.Y.; Henry, M.; Soulet, D.; Hébert, S.S.; Toth, K.; Mouginot, D.; Drolet, G. Extracellular Na+ levels regulate formation and activity of the NaX/alpha1-Na+/K+-ATPase complex in neuronal cells. Front. Cell Neurosci. 2014, 8, 413. [Google Scholar] [CrossRef]
- Graf, J.; Petersen, O.H. Cell membrane potential and resistance in liver. J. Physiol. 1978, 284, 105–126. [Google Scholar] [CrossRef]
- De Hert, S.; De Baerdemaeker, L.; De Maeseneer, M. What the phlebologist should know about local anesthetics. Phlebology 2014, 29, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: A reassessment and a hypothesis. Am. J. Physiol. Cell Physiol. 1977, 232, C165–C173. [Google Scholar] [CrossRef]
- Huntington, J.A. How Na+ activates thrombin–a review of the functional and structural data. Biol. Chem. 2008, 389, 1025–1035. [Google Scholar] [CrossRef]
- Minta, A.; Tsien, R.Y. Fluorescent indicators for cytosolic sodium. J. Biol. Chem. 1989, 264, 19449–19457. [Google Scholar] [PubMed]
- Szmacinski, H.; Lakowicz, J.R. Sodium Green as a Potential Probe for Intracellular Sodium Imaging Based on Fluorescence Lifetime. Anal. Biochem. 1997, 250, 131–138. [Google Scholar] [CrossRef]
- Meier, S.D.; Kovalchuk, Y.; Rose, C.R. Properties of the new fluorescent Na+ indicator CoroNa Green: Comparison with SBFI and confocal Na+ imaging. J. Neurosci. Methods 2006, 155, 251–259. [Google Scholar] [CrossRef]
- Kim, M.K.; Lim, C.S.; Hong, J.T.; Han, J.H.; Jang, H.-Y.; Kim, H.M.; Cho, B.R. Sodium-Ion-Selective Two-Photon Fluorescent Probe for In Vivo Imaging. Angew. Chem. Int. Ed. 2010, 49, 364–367. [Google Scholar] [CrossRef]
- Sarkar, A.R.; Heo, C.H.; Park, M.Y.; Lee, H.W.; Kim, H.M. A small molecule two-photon fluorescent probe for intracellular sodium ions. Chem. Commun. 2014, 50, 1309–1312. [Google Scholar] [CrossRef]
- Baron, S.; Caplanusi, A.; van de Ven, M.; Radu, M.; Despa, S.; Lambrichts, I.; Ameloot, M.; Steels, P.; Smets, I. Role of Mitochondrial Na+ Concentration, Measured by CoroNa Red, in the Protection of Metabolically Inhibited MDCK Cells. JASN 2005, 16, 3490–3497. [Google Scholar] [CrossRef]
- Wieland, T.; Faulstich, H. Amatoxins, phallotoxins, phallolysin, and antamanide: The biologically active components of poisonous Amanita mushrooms. Crc. Crit. Rev. Biochem. 1978, 5, 185–260. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.O.; Carrell, C.J.; Bush, L.A.; Prasad, S.; Caccia, S.; Chen, Z.-W.; Mathews, F.S.; Di Cera, E. Molecular Dissection of Na+ Binding to Thrombin. J. Biol. Chem. 2004, 279, 31842–31853. [Google Scholar] [CrossRef]
- Juers, D.H.; Rob, B.; Dugdale, M.L.; Rahimzadeh, N.; Giang, C.; Lee, M.; Matthews, B.W.; Huber, R.E. Direct and indirect roles of His-418 in metal binding and in the activity of β-galactosidase (E. coli). Protein Sci. 2009, 18, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, S.; Gaillard, T.; Rineau, E. Iron is essential for living! Crit. Care 2014, 18, 678. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J. Free manganese (II) and iron (II) cations can act as intracellular cell controls. FEBS Lett. 1982, 140, 3–10. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E. Interactions of Iron with Reactive Intermediates of Oxygen and Nitrogen. Dev. Neurosci 2002, 24, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.P.; Ryde, U. How O2 Binds to Heme: Reasons for rapid binding and spin inversion. J. Biol. Chem. 2004, 279, 14561–14569. [Google Scholar] [CrossRef]
- Gilardi, G.; Di Nardo, G. Heme iron centers in cytochrome P450: Structure and catalytic activity. Rend. Fis. Acc. Lincei 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins containing cytochrome, iron-sulfur, or copper redox centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.M.; Ludden, P.W. Biosynthesis of the Iron-Molybdenum Cofactor of Nitrogenase. Annu. Rev. Microbiol. 2008, 62, 93–111. [Google Scholar] [CrossRef] [Green Version]
- Schick, M.; Xie, X.; Ataka, K.; Kahnt, J.; Linne, U.; Shima, S. Biosynthesis of the iron-guanylylpyridinol cofactor of [Fe]-hydrogenase in methanogenic archaea as elucidated by stable-isotope labeling. J. Am. Chem. Soc. 2012, 134, 3271–3280. [Google Scholar] [CrossRef]
- Sanvisens, N.; Bañó, M.C.; Huang, M.; Puig, S. Regulation of Ribonucleotide Reductase in Response to Iron Deficiency. Mol. Cell 2011, 44, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, A.; Sawada, H.; Kato, T.; Nakayama, T.; Yamamoto, H.; Matsumoto, Y. Purification and characterization of a purple acid phosphatase from rat spleen. J. Biochem. 1984, 95, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Spanner, M.; Weber, K.; Lanske, B.; Ihbe, A.; Siggelkow, H.; Schütze, H.; Atkinson, M.J. The iron-binding protein ferritin is expressed in cells of the osteoblastic lineage in vitro and in vivo. Bone 1995, 17, 161–165. [Google Scholar] [CrossRef]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease: Iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef]
- García-Beltrán, O.; Mena, N.; Yañez, O.; Caballero, J.; Vargas, V.; Nuñez, M.T.; Cassels, B.K. Design, synthesis and cellular dynamics studies in membranes of a new coumarin-based “turn-off” fluorescent probe selective for Fe2+. Eur. J. Med. Chem. 2013, 67, 60–63. [Google Scholar] [CrossRef]
- Wang, R.; Yu, F.; Liu, P.; Chen, L. A turn-on fluorescent probe based on hydroxylamine oxidation for detecting ferric ion selectively in living cells. Chem. Commun. 2012, 48, 5310. [Google Scholar] [CrossRef]
- Breuer, W.; Epsztejn, S.; Millgram, P.; Cabantchik, I.Z. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am. J. Physiol. Cell Physiol. 1995, 268, C1354–C1361. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Umar, S.; Nag, P.; Sharma, A.; Kumar, L.; Shamsuzzama, S.; Hossain, Z.; Gayen, J.R.; Nazir, A. A dual colorimetric-ratiometric fluorescent probe NAP-3 for selective detection and imaging of endogenous labile iron(III) pools in C. elegans. Chem. Commun. 2015, 51, 5001–5004. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fang, L.; Zhou, H.; Zhang, W.; Wang, X.; Li, N.; Zhong, H.; Tang, B. A New Ratiometric Fluorescent Probe for Detection of Fe2+ with High Sensitivity and Its Intracellular Imaging Applications. Chem. Eur. J. 2011, 17, 10520–10523. [Google Scholar] [CrossRef]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef]
- Foster, A.W.; Osman, D.; Robinson, N.J. Metal Preferences and Metallation. J. Biol. Chem. 2014, 289, 28095–28103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.R.; Fernandes, J.; Go, Y.-M.; Jones, D.P. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun. 2017, 482, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, J.D.; Culotta, V.C. Battles with Iron: Manganese in Oxidative Stress Protection. J. Biol. Chem. 2012, 287, 13541–13548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S.; Bachert, C.; Smith, D.R.; Linstedt, A.D. Manganese-induced Trafficking and Turnover of the cis -Golgi Glycoprotein GPP130. MBoC 2010, 21, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A. Manganese action in brain function. Brain Res. Brain Res. Rev. 2003, 41, 79–87. [Google Scholar] [CrossRef]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Canary, J.W. Discrimination between Hard Metals with Soft Ligand Donor Atoms: An On-Fluorescence Probe for Manganese(II). Angew. Chem. Int. Ed. 2010, 49, 7710–7713. [Google Scholar] [CrossRef]
- Guth, J.H.; Burris, R.H. The role of Mg2+ and Mn2+ in the enzyme-catalysed activation of nitrogenase Fe protein from Rhodospirillum rubrum. Biochem. J. 1983, 213, 741–749. [Google Scholar] [CrossRef]
- Kehres, D.G.; Maguire, M.E. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol. Rev. 2003, 27, 263–290. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, C.; Grodsky, N.B.; Ariyaratne, N.; Colman, R.F.; Bahnson, B.J. Crystal Structure of Porcine Mitochondrial NADP+ -dependent Isocitrate Dehydrogenase Complexed with Mn2+ and Isocitrate: INSIGHTS INTO THE ENZYME MECHANISM. J. Biol. Chem. 2002, 277, 43454–43462. [Google Scholar] [CrossRef]
- Romero, N.; Benítez, J.; Garcia, D.; González, A.; Bennun, L.; García-Robles, M.A.; López, V.; Wilson, L.A.; Schenk, G.; Carvajal, N.; et al. Mammalian agmatinases constitute unusual members in the family of Mn2+ -dependent ureahydrolases. J. Inorg. Biochem. 2017, 166, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Thestrup, T.; Litzlbauer, J.; Bartholomäus, I.; Mues, M.; Russo, L.; Dana, H.; Kovalchuk, Y.; Liang, Y.; Kalamakis, G.; Laukat, Y.; et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat. Methods 2014, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Craggs, T.D. Green fluorescent protein: Structure, folding and chromophore maturation. Chem. Soc. Rev. 2009, 38, 2865–2875. [Google Scholar] [CrossRef]





| GEP | Localization | KD In Vitro/In Situ | Dynamic Range | λExc (nm) | λEm (nm) | Ref. |
|---|---|---|---|---|---|---|
| GEPII 1.0 | Cytosol, Nucleus, Mitochondria, Subplasma membrane * | 420 µM/820 µM | 220% | 430 | 475/525 | [30] |
| lc-LysM GEPII 1.0 | 30.47 mM/60.95 mM | 80% | 430 | 475/525 | [30] | |
| GEPII 2.7 | Cytosol | 3.24 mM/9.09 mM | 110% | 430 | 475/525 | [30] |
| GEPII 2.10 | 4.39 mM/10.11 mM | 150% | 430 | 475/525 | [30] | |
| GEPII 2.15 | 8.59 mM/15.59 mM | 150% | 430 | 475/525 | [30] | |
| lc-LysM R-GEPII 1.0 | -/75.12 mM | 30% | 480 | 510/560 | Unpublished | |
| R-GEPII 1.0 | -/3.25 mM | 20% | 477 | 510/560 | Unpublished | |
| KIRIN1 | 1.66 mM/- | 130% | 430 | 475/525 | [66] | |
| KIRIN1-GR | 2.56 mM/- | 20% | 480 | 510/560 | [66] |
| GEP | Localization | KD | Dynamic Range | λExc (nm) | λEm (nm) | Ref. |
|---|---|---|---|---|---|---|
| MagFRET-1 | Cytosol | 150 µM | 49% | 430 | 475/525 | [34] |
| MagFRET-2 | 350 µM | 33% | 430 | 475/525 | [34] | |
| MagFRET-3 | 9.2 mM | 58% | 430 | 475/525 | [34] | |
| MagFRET-4 | 8.5 mM | 62% | 430 | 475/525 | [34] | |
| MagFRET-5 | 7.4 mM | 74% | 430 | 475/525 | [34] | |
| MagFRET-6 | 15 mM | 50% | 430 | 475/525 | [34] | |
| MagFRET-7 | 780 µM | 38% | 430 | 475/525 | [34] | |
| MagFRET-8 | 890 µM | 56% | 430 | 475/525 | [34] | |
| MARIO | 7.2 mM | 153% | 430 | 475/525 | [88] | |
| MagFRET-1 NLS | Nucleus | 150 µM | 49% | 430 | 475/525 | [34] |
| NLS-MARIO | 7.2 mM | 153% | 430 | 475/525 | [88] |
| GEP | Localization | KD | Dynamic Range | λExc (nm) | λEm (nm) | Ref. |
|---|---|---|---|---|---|---|
| YC2.1 | Cytosol | 100 nM and 4.3 µM | 2 | 430 | 475/525 | [27,129] |
| YC3.1 | 1.5 µM | 2 | 430 | 475/525 | [27,129] | |
| YC3.3 | 1.5 µM | ~ 1.1 | 430 | 475/525 | [27,115] | |
| YC6.1 | 110 nM | 2 | 430 | 475/525 | [116] | |
| YC3.60 | 250 nM | 560% | 430 | 475/525 | [117] | |
| D1 | 800 nM and 60 µM | - | 430 | 475/525 | [118] | |
| D2cpV | 30 nM and 3 µM | 5.3 | 430 | 475/525 | [119] | |
| D3cpV | 600 nM | 5.1 | 430 | 475/525 | [119] | |
| D4cpV | 64 µM | 3.8 | 430 | 475/525 | [119] | |
| TN-humcTnC | 470 nM | 100% | 430 | 475/525 | [123] | |
| TN-L15 | 1.2 µM | 100% | 430 | 475/525 | [123] | |
| TN-XL | 2.5 µM | 400% | 430 | 475/525 | [124] | |
| LynD3cpV | Subplasma-membrane | 600 nM | 5.1 | 430 | 475/525 | [119] |
| Cav2.2-TN-XL | 2.5 µM | - | 430 | 475/525 | [122] | |
| TN-L15D107ARas | 29 µM | 100% | 430 | 475/525 | [123] | |
| H2BD1cpV | Nucleus | 800 nM and 60 µM | - | 430 | 475/525 | [120] |
| 4mtD3cpV | Mitochondria | 600 nM | 5.1 | 430 | 475/525 | [119] |
| 4mtD1GO-Cam | Mitochondria | 1.53 µM | - | 477 | 510/560 | [48] |
| N33D1cpV | Outer mitochondrial-membrane | 800 nM and 60 µM | - | 430 | 475/525 | [120] |
| Split YC7.3er | Endoplasmic Reticulum | 130 µM | - | 430 | 475/525 | [121] |
| D1ER | 220 µM | - | 430 | 475/525 | [118] | |
| D1ERCmR2 | 200 µM | - | 480 | 510/560 | [32] | |
| apoK1-er | 124 µM | - | 430 | 475/525 | [126] | |
| YC4.3ER | 800 nM and 700 µM | - | 430 | 475/525 | [118] |
| GEP | Localization | KD | Dynamic Range | λExc (nm) | λEm (nm) | Ref. |
|---|---|---|---|---|---|---|
| Amt1-FRET | Cytosol | 2.5 aM | - | 430 | 475/525 | [36] |
| Ace1-FRET | 4.7 aM | - | 430 | 475/525 | [142] | |
| Mac1-FRET | 97 zM | - | 430 | 475/525 | [142] | |
| eCALWY-C2M/C3M | - | - | 430 | 475/525 | [153] | |
| PMtb-FRET | - | 3.31 zM | - | 430 | 475/525 | [146] |
| GEP | Localization | KD | Dynamic Range | λExc (nm) | λEm (nm) | Ref. |
|---|---|---|---|---|---|---|
| CA + WY | Cytosol | 350 pM | - | 430 | 475/525 | [35] |
| Cys2His2 | Cytosol, Mitochondria * | 1.7 µM | 2.2 | 430 | 475/525 | [163] |
| His4 | Cytosol, Mitochondria, Plasma membrane * | 160 µM | 4 | 430 | 475/525 | [163] |
| eCALWY-1 | Cytosol, Insulin-storing granules * | 2 pM | 2 | 430 | 475/525 | [159] |
| eCALWY-2 | Cytosol | 9 pM | 2 | 430 | 475/525 | [159] |
| eCALWY-3 | Cytosol | 45 pM | 1.7 | 430 | 475/525 | [159] |
| eCALWY-4 | Cytosol | 630 pM | 2 | 430 | 475/525 | [159] |
| eCALWY-5 | Cytosol | 1.8 nM | 1.8 | 430 | 475/525 | [159] |
| eCALWY-6 | Cytosol, Insulin-storing granules * | 2.9 nM | 1.8 | 430 | 475/525 | [159] |
| ZinCh-6 | - | 260 nM | ~ 3.5 | 430 | 475/525 | [168] |
| ZinCh-9 | - | 500 nM and 88 µM | 4 | 430 | 475/525 | [168] |
| ZapCY1 | ER, Golgi Apparatus * | 2.5 pM | 4.15 | 430 | 475/525 | [170] |
| ZapSM2 | Cytosol, Nucleus * | - | 1.1 | 400 | 510/560 | [171] |
| ZapSR2 | Cytosol, Nucleus * | - | 1.2 | 400 | 510/580 | [171] |
| ZapOC2 | Cytosol, Nucleus * | - | 1.1 | 550 | 565/610 | [171] |
| ZapOK2 | Cytosol, Nucleus * | - | 1.1 | 550 | 565/635 | [171] |
| ZapCmR1 | Cytosol, Nucleus * | - | 1.15 | 480 | 510/560 | [171] |
| ZapCmR1.1 | Cytosol, Nucleus * | - | 1.5 | 480 | 510/560 | [171] |
| ZapCmR2 | Cytosol, Nucleus * | - | 1.4 | 480 | 510/560 | [171] |
| eZinCh-2 | Cytosol, Mitochondria, ER, Vesicles * | 1 nM | 300% | 430 | 475/525 | [169] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischof, H.; Burgstaller, S.; Waldeck-Weiermair, M.; Rauter, T.; Schinagl, M.; Ramadani-Muja, J.; Graier, W.F.; Malli, R. Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes. Cells 2019, 8, 492. https://doi.org/10.3390/cells8050492
Bischof H, Burgstaller S, Waldeck-Weiermair M, Rauter T, Schinagl M, Ramadani-Muja J, Graier WF, Malli R. Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes. Cells. 2019; 8(5):492. https://doi.org/10.3390/cells8050492
Chicago/Turabian StyleBischof, Helmut, Sandra Burgstaller, Markus Waldeck-Weiermair, Thomas Rauter, Maximilian Schinagl, Jeta Ramadani-Muja, Wolfgang F. Graier, and Roland Malli. 2019. "Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes" Cells 8, no. 5: 492. https://doi.org/10.3390/cells8050492
APA StyleBischof, H., Burgstaller, S., Waldeck-Weiermair, M., Rauter, T., Schinagl, M., Ramadani-Muja, J., Graier, W. F., & Malli, R. (2019). Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes. Cells, 8(5), 492. https://doi.org/10.3390/cells8050492