Abstract
Background
The diagnostic utility of prostate biopsy is limited for prostate cancer (PCa) in the prostate-specific antigen (PSA) grey zone. This study aims to evaluate the diagnostic performance of multiparametric magnetic resonance imaging (mpMRI) and prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) for PSA grey zone PCa and clinically significant PCa (csPCa).
Methods
A total of 82 patients with PSA levels ranging from 4 to 10 ng/mL who underwent 18F-PSMA-1007 PET/CT, mpMRI, and prostate biopsy were prospectively enrolled. For 18F-PSMA-1007 PET/CT and mpMRI in detecting PCa and csPCa, sensitivity, specificity, and area under the curve (AUC) were assessed using biopsy histology as the standard.
Results
18F-PSMA-1007 PET/CT demonstrated better diagnostic performance for PCa than mpMRI (AUC 0.81 vs. 0.63, P = 0.02). 11.0% of patients with PI-RADS 3-5 had no PCa on biopsy, of whom 77.8% were correctly differentiated by 18F-PSMA-1007 PET/CT. Combined 18F-PSMA-1007 PET/CT + mpMRI improved sensitivity (92.5% vs. 73.6%) and negative predictive value (NPV, 78.9% vs. 53.3%) compared with mpMRI alone.
Conclusions
18F-PSMA-1007 PET/CT outperformed mpMRI for detecting PCa in the grey zone level of PSA. 18F-PSMA-1007 PET/CT in combination with mpMRI has additional improvement in sensitivity and NPV for csPCa detection.
Clinical Trial Registration: NCT05958004, 2024-07.

Similar content being viewed by others
Introduction
With the widespread serum prostate-specific antigen (PSA) tests, the number of prostate cancer (PCa) cases has risen rapidly in the last few decades [1]. However, an elevated PSA level could be caused by PCa and some non-malignant conditions, and the low specificity of the PSA screening may lead to potential overdiagnosis and overtreatment [2, 3]. Some patients will undergo unnecessary biopsies due to a false-positive PSA [4]. Moreover, the PCa detection rate of those with grey zone PSA levels ranging from 4 to 10 ng/mL on biopsy is between 16 and 39%, and the clinically significant prostate cancer (csPCa) detection rate is much lower [5]. Additionally, there is a significant probability of inaccurate samples and complications using biopsy, and it is difficult to discriminate between indolent and aggressive disorders [6, 7]. These obstacles prompted a growing interest in noninvasive and visual alternatives for early diagnosis of PCa and csPCa within PSA grey zone level, as well as reducing unneeded biopsies.
Multiparametric MRI (mpMRI) is an established imaging modality to obtain better sensitivity in the detection and localisation of csPCa and avoid biopsy [8, 9]. MRI-based prediction models that integrate clinical characteristics have demonstrated high predictive ability, and the Prostate Imaging Reporting and Data System (PI-RADS) score is one of the reliable variables for the diagnosis of PCa and csPCa in the PSA grey zone [10]. Nevertheless, mpMRI exhibits a low positive predictive value (PPV) for csPCa ranging from 34 to 68% [9, 11], and remains difficult to characterise PI-RADS 3 lesions, resulting in unnecessary biopsies. Prostate-specific membrane antigen (PSMA) PET/CT has been proven to outperform conventional imaging in detecting primary PCa [12,13,14,15], and it is an alternative and promising method to detect csPCa missed by mpMRI [16]. Studies have investigated the potential of PSMA PET/CT for the detection of intermediate- to high-risk intraprostatic tumour lesions [17,18,19], while the utility of detecting PSA grey zone PCa and csPCa in the pre-biopsy setting is uncertain. Therefore, this study evaluates the diagnostic accuracy of PSMA PET/CT in detecting PCa and csPCa in the PSA grey zone compared to mpMRI.
Patients and methods
Patients
Men with initial PSA levels of 4–10 ng/mL from June 2023 to March 2024 were consecutively enrolled in this prospective registered clinical trial (NCT 05958004). All recruited patients underwent prostate mpMRI, 18F-PSMA-1007 PET/CT, and prostate biopsy. The exclusion criteria included (1) previously known diagnosis of PCa; (2) history of other active malignancy; and (3) any absolute contra-indication to prostate mpMRI. This study was approved by the hospital ethics committee (No. KYLLSL-2023-248), and informed consent was obtained from all individual participants. Data from the research were collected and managed using Research Electronic Data Capture (REDCap) tools.
mpMRI imaging acquisition and analysis
All candidates underwent prostate mpMRI performed in a 3.0-T scanner. The mpMRI protocol included T1-weighted images, T2-weighted images, diffusion-weighted images, and dynamic-contrast enhanced images. Abnormalities were reported by two experienced prostate radiologists who were unaware of clinical and pathological results according to PI-RADS version 2.1 [20]. Concordant conclusions regarding the abnormalities of mpMRI findings were required of the 2 readers. Positive mpMRI was defined as PI-RADS ≥ 3.
PSMA PET/CT imaging acquisition
18F-PSMA-1007 was synthesised as described previously [21] and injected intravenously at a dose of 3.7 MBq/kg body weight. Patients underwent 18F-PSMA-1007 PET/CT scans 90 min after injection. PET attenuation was determined using low-dose CT scans from the head to the proximal thighs (pitch 0.8 mm, 60 mA, 140 kV [peak]) with 512 × 512 matrices. Whole-body PET scans were performed in three dimensions (emission time: 90 seconds per bed position, scanned at 7–10 beds) [21].
PSMA PET/CT analysis and intra‑ and inter-observer variability analysis
Two qualified nuclear medicine physicians, blinded to the mpMRI and clinical outcomes, independently reviewed all PET/CT scans. A third read was performed to assess for discrepancies. The intraprostatic lesions were reviewed semi-quantitatively using the maximum standardised uptake value (SUVmax), based on the cutoff from our previous study [21] (PSMA-negative: SUVmax < 8.3, and PSMA-positive: SUVmax > 8.3), and also reported qualitatively based on their clinical experience using a four-point certainty scale (classifying “probably or definitely positive” as positive and “probably or definitely negative” as negative) [22]. 18F-PSMA-1007 PET/CT positivity was defined as either quantitatively positive and/or qualitatively positive.
Each observer re-assessed all cases at least two months following the first assessments to ascertain the intra-observer agreement in differentiating between PCa and non-PCa. The observers were blinded to their prior evaluations. The inter-observer variability of distinguishing between PCa and non-PCa was determined by comparing the consistency among observers.
Prostate biopsy
An experienced urologist performed transperineal prostate systematic biopsies with a minimum of 12 cores. When possible, additional _targeted biopsies were performed for patients with positive findings on mpMRI and/or 18F-PSMA-1007 PET/CT using a fusion biopsy system. The urologist will consider the key images to demonstrate the sites of positive lesions prior to biopsy. A maximum of three suspected lesions will be _targeted, with one to three cores per lesion.
A specialised uropathologist assessed and interpreted each prostate biopsy in accordance with the guidelines established by the International Society of Urological Pathology (ISUP). The ISUP grade group of positive biopsies was documented. ISUP ≥ 2 indicates csPCa.
Statistical analysis
The diagnostic value of mpMRI and 18F-PSMA-1007 PMSA PET/CT was evaluated using sensitivity, specificity, PPV, negative predictive value (NPV), and area under the curve (AUC). The performance of mpMRI (PI-RADS) and 18F-PSMA-1007 PMSA PET/CT (SUVmax) in differentiating between csPCa and non-csPCa lesions was assessed using a receiver operating characteristic (ROC) curve. To maximise sensitivity and specificity, the best cutoff value was determined using the Youden method. AUCs were compared using the DeLong test. Cohen’s weighted kappa method was used to estimate both intra- and inter-observer variability. Kappa values of 0 to 0.20 suggest weak agreement, 0.21 to 0.40 - fair agreement, 0.41 to 0.60 - moderate agreement, 0.61 to 0.80 - substantial agreement, and 0.81 to 1.0 - perfect agreement. Statistical analysis was carried out using MedCalc software (19.0.4). P < 0.05 was considered statistically significant.
Results
Participant characteristics
From June 2023 to March 2024, 92 patients were prospectively recruited (Fig. 1). Of those, 2 patients without complete prostate biopsy and 8 patients without complete mpMRI were excluded due to the presence of metal implants or foreign bodies. The study comprised 82 participants with a median age of 67 years (IQR 62-72 years) who completed both imaging modalities and biopsy. Baseline characteristics are described in Table 1. The median time interval from mpMRI to 18F-PSMA-1007 PET/CT was 15 days (IQR 9–24 d). And the median PSA level was 7.9 ng/mL (IQR 6.5–9.4). Non-PCa were detected in 14 (17.1%) patients by biopsy histology, and PCa was detected in 68 (82.9%) patients, including 53 (64.6%) with csPCa (ISUP ≥ 2) and 15 (18.3%) with non-csPCa (ISUP 1).
Diagnostic performance of mpMRI and PSMA PET/CT
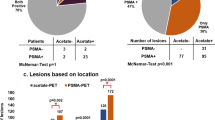
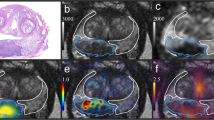
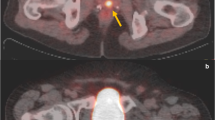
Of the included 82 patients, 71 (86.8%) were classified as PCa based on mpMRI (PI-RADS of 3–5), 54 (65.9%) were considered positive on 18F-PSMA-1007 PET/CT, and 46 (56.1%) were positive on both modalities. Of the 68 biopsy-proven PCa patients, 6 (8.8%) were PSMA positive with PI-RADS 1-2 (Fig. 2), while 16 (23.5%) were PSMA negative with PI-RADS 3-5 (Fig. 3). Of the remaining 14 non-PCa patients, 9 (11.1%) were had PI-RADS ≥ 3, of whom 7 (77.8%) were PSMA negative (Fig. 4).
The proportion of men with csPCa and a PI-RADS of 1, 2, 3, 4, or 5 was 1/6, 3/5, 10/19, 31/42, and 8/10, respectively. Among men with positive 18F-PSMA-1007 PET/CT, 64.8% (35/54) had csPCa.
The diagnostic value of both imaging modalities for PCa with biopsy histology as the standard is shown in Table 2. The AUC of mpMRI alone was 0.63 (95% CI 0.52, 0.74), with sensitivity, specificity, PPV and NPV of 91.2% (95% CI 81.8%, 96.7%), 35.7% (95% CI 12.8%, 64.9%), 87.3% (95% CI 82.2%, 91.1%) and 45.5% (95% CI 22.8%, 70.2%), respectively. For 18F-PSMA-1007 PET/CT, the AUC was 0.81 (95% CI 0.71, 0.89), with sensitivity of 76.5% (95% CI 64.6%, 85.9%), specificity of 85.7% (95% CI 57.2%, 98.2%), PPV of 96.3% (95% CI 87.7%, 98.6%) and NPV of 42.9% (95% CI 31.7%, 54.7%). 18F-PSMA-1007 PET/CT showed greater performance than that of mpMRI (AUC 0.81 vs. 0.63, P = 0.02).
For predicting csPCa, the AUC of mpMRI alone was 0.67 (95% CI 0.56, 0.77). The Youden-selected threshold of PI-RADS was 4. PI-RADS 4-5 vs. 1-3 had a specificity of 55.2% (95% CI 35.7%, 73.6%) and an NPV of 53.3% (95% CI 39.6%, 66.6%). The AUC of 18F-PSMA-1007 PET/CT alone was 0.71 (95% CI 0.60, 0.80), which is higher than that of mpMRI but not statistically significant (P = 0.58). A SUVmax cutoff of 9.3 showed an improvement in specificity (72.4% vs. 55.2%) and PPV (80.9% vs. 75.0%) compared with mpMRI, but the sensitivity (64.2% vs. 73.6%) and NPV (52.5% vs. 53.3%) were lower. Combined 18F-PSMA-1007 PET/CT + mpMRI showed a rise in NPV compared with mpMRI alone (78.9% vs. 53.3%, Table 3). The combination also improved sensitivity (92.5% vs. 73.6%), although specificity was slightly reduced (51.7% vs. 55.2%).
Observer variability evaluation
All 18F-PSMA-1007 PET/CT scans were re-assessed, with results available for evaluation of inter- and intra-observer variability, respectively. In terms of predicting PCa, the kappa value of 0.64 (95% CI 0.46, 0.82) between the two observers suggested a substantial inter-observer agreement. Similarly, an average kappa value of 0.66 (95% CI 0.47, 0.85) indicated a substantial intra-observer agreement.
Discussion
Diagnosis of PCa is critical for treatment decisions and prognosis outcomes, particularly in individuals within PSA grey zone levels. The low positive rate of biopsy for individuals with PCa and csPCa in PSA grey zone levels has resulted in underdiagnosis in some cases. A prospective clinical trial found that mpMRI could increase the rate of detecting csPCa on biopsy and decrease the number of biopsies performed [23]. Our study found that 18F-PSMA-1007 PET/CT had greater diagnostic accuracy than mpMRI for PCa and csPCa within PSA grey zone levels, although the difference is not significant in csPCa detection. Furthermore, combined 18F-PSMA-1007 PET/CT + mpMRI increased sensitivity and NPV for csPCa within PSA grey zone levels compared to mpMRI alone, potentially identifying individuals who could safely avoid biopsy.
Several studies have directly investigated the efficacy of 18F-PSMA-1007 PET/CT and mpMRI for diagnosing PCa and csPCa [22, 24]. A prospective single-arm trial [24] reported that mpMRI had greater diagnostic accuracy than 68Ga-PSMA-11 PET/CT for diagnosing PCa. The current study indicated that 18F-PSMA-1007 PET/CT had a similar lower sensitivity but higher specificity, probably due to the differences in PSA levels and radiotracers. The compelling benefits of 18F-PSMA-1007 PET/CT in this trial were in patients with positive mpMRI. 11.0% of patients with PI-RADS 3-5 had no PCa on biopsy, of whom 77.8% correctly differentiated by negative 18F-PSMA-1007 PET/CT. In addition, 56.1% of patients with positive mpMRI had positive 18F-PSMA-1007 PET/CT, of whom 95.8% had PCa on biopsy. Furthermore, 18F-PSMA-1007 PET/CT has certain advantages to mpMRI. First, PET/CT is regarded as a one-stop-shop scan [25], with scans ranging from the head to the proximal thighs, providing information on both primary and metastatic lesions. Second, mpMRI is not feasible for patients with metal implants, and 18F-PSMA-1007 PET/CT should be considered for this group of patients. Finally, the characteristics of PI-RADS 3 lesions in mpMRI are hard to determine. It has been demonstrated that PSMA PET/CT could be capable of stratifying PI-RADS 3 lesions on mpMRI [26, 27]. However, mpMRI will remain important in the primary setting because of its higher spatial resolution and the potential of false-positive PSMA PET [28].
18F-PSMA-1007 PET/CT and mpMRI both had a low NPV for csPCa within PSA grey zone levels. However, the synergistic impact of combining 18F-PSMA-1007 PET/CT and mpMRI was validated, and both sensitivity and NPV were better than 18F-PSMA-1007 PET/CT alone and mpMRI alone. Similarly, the PRIMARY study [22] found that combining 68Ga-PSMA-11 PET/CT with mpMRI increased sensitivity and NPV for the detection of csPCa. Given the high sensitivity and NPV of 18F-PSMA-1007 PET/CT + mpMRI in detecting csPCa, it may be safe to skip biopsy in patients who have negative 18F-PSMA-1007 PET/CT + mpMRI findings.
In this study, inter- and intra-observer agreement was substantial for detecting PCa. Similarly, Wondergem et al. [29] found a substantial interreader agreement (0.74) for 18F-PSMA-1007 PET/CT assessment of PCa. However, some variability was present in both inter-observer and intra-observer agreements, which may be related to the lack of appropriate reporting guidelines for intraprostatic lesions on 18F-PSMA-1007 PET/CT. Further improvement in clinical reporting criteria needs to be done to add the reliability of intraprostatic lesion detection across centres with varied equipment. In addition, integrating machine learning methods into 18F-PSMA-1007 PET/CT images could have the potential to increase diagnostic accuracy and consistency for detecting PSA grey zone PCa and csPCa.
There are certain limitations to our investigation. To begin, this trial used prostate biopsy histology rather than prostatectomy pathology to determine PCa and csPCa, which may have resulted in false-negative results or inaccurate Gleason score classification due to sampling error. To reduce the influence on our investigation, systematic biopsy in conjunction with mpMRI and/or 18F-PSMA-1007 PET/CT-based _targeted biopsy was required for each case. Second, this is a single-centre study. Although all participants were enrolled and tested prospectively, the comparative analysis of 18F-PSMA-1007 PET/CT and mpMRI for PCa detection would have been influenced by the small sample of non-PCa. Third, 60% (3/5) of PI-RADS 2 lesions in this study were pathologically proven csPCa, a higher proportion compared with previous studies [22, 24], which could be related to the fact that there were only five PI-RADS 2 lesions in total. Therefore, larger sample sizes would be required to further validate the outcomes of the study. Fourth, our study did not carry out a lesion-based analysis, similar to the PRIMARY trial [22]. This is a trial to identify PCa and csPCa using 18F-PSMA-1007 PET/CT and mpMRI, and the findings may pave the way for future trials investigating the additional value of 18F-PSMA-1007 PET/CT over mpMRI in the detection of PSA grey zone PCa and csPCa.
In conclusion, 18F-PSMA-1007 PET/CT demonstrated greater diagnostic efficacy than mpMRI for the detection of PCa in the PSA grey zone, while not significantly higher when detecting csPCa in the PSA grey zone. Combined 18F-PSMA-1007 PET/CT + mpMRI has additional improvement in sensitivity and NPV. The inter-observer and intra-observer agreement of diagnosing PCa on 18F-PSMA-1007 PET/CT was substantial. 18F-PSMA-1007 PET/CT could be used for patients within PSA grey zone levels who are unable to undergo mpMRI.
Data availability
The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request.
References
Pinsky PF, Parnes H. Screening for prostate cancer. N Engl J Med. 2023;388:1405–14.
Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319:1901–13.
Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519.
Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388:1547–58.
Aminsharifi A, Howard L, Wu Y, De Hoedt A, Bailey C, Freedland SJ, et al. Prostate specific antigen density as a predictor of clinically significant prostate cancer when the prostate specific antigen is in the diagnostic gray zone: defining the optimum cutoff point stratified by race and body mass index. J Urol. 2018;200:758–66.
Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92.
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71:353–65.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 Update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Li X, Li C, Chen M. Patients With “Gray Zone” PSA levels: application of prostate MRI and MRS in the diagnosis of prostate cancer. J Magn Reson Imaging. 2023;57:992–1010.
Thompson JE, van Leeuwen PJ, Moses D, Shnier R, Brenner P, Delprado W, et al. The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. J Urol. 2016;195:1428–35.
Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Metaanalysis of (68)Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60:786–93.
Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F. et al. 68)Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21:204–11.
Donato P, Roberts MJ, Morton A, Kyle S, Coughlin G, Esler R, et al. Improved specificity with (68)Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging. 2019;46:20–30.
Rhee H, Thomas P, Shepherd B, Gustafson S, Vela I, Russell PJ, et al. Prostate specific membrane antigen positron emission tomography may improve the diagnostic accuracy of multiparametric magnetic resonance imaging in localized prostate cancer. J Urol. 2016;196:1261–7.
Donato P, Roberts M, Morton A, Kyle S, Coughlin G, Esler R, et al. Improved specificity with Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nuclear Med Mol Imaging. 2019;46:20–30.
Chow KM, So WZ, Lee HJ, Lee A, Yap DWT, Takwoingi Y, et al. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: a systematic review and meta-analysis. Eur Urol. 2023;84:36–48.
Scheltema MJ, Chang JI, Stricker PD, van Leeuwen PJ, Nguyen QA, Ho B, et al. Diagnostic accuracy of (68) Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: impact of the addition of (68) Ga-PSMA PET to mpMRI. BJU Int. 2019;124:42–49.
Mookerji N, Pfanner T, Hui A, Huang G, Albers P, Mittal R, et al. Fluorine-18 prostate-specific membrane antigen-1007 PET/CT vs multiparametric MRI for locoregional staging of prostate cancer. JAMA Oncol. 2024;10:1097–103.
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system Version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–51.
Luo L, Zheng A, Chang R, Li Y, Gao J, Wang Z, et al. Evaluating the value of (18)F-PSMA-1007 PET/CT in the detection and identification of prostate cancer using histopathology as the standard. Cancer Imaging. 2023;23:108.
Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the Diagnosis of Prostate Cancer (PRIMARY): a prospective multicentre study. Eur Urol. 2021;80:682–9.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-_targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77.
Wong LM, Sutherland T, Perry E, Tran V, Spelman T, Corcoran N et al. Fluorine-18-labelled prostate-specific membrane antigen positron emission tomography/computed tomography or magnetic resonance imaging to diagnose and localise prostate cancer. A Prospective Single-arm Paired Comparison (PEDAL). Eur Urol Oncol 2024. https://doi.org/10.1016/j.euo.2024.01.002.
Walz J. Better to rule in or rule out significant prostate cancer? The added value of prostate-specific membrane antigen positron emission tomography to magnetic resonance imaging diagnostic pathways for prostate cancer. Eur Urol Oncol. 2022;5:401–2.
Yang J, Tang Y, Zhou C, Zhou M, Li J, Hu S. The use of (68) Ga-PSMA PET/CT to stratify patients with PI-RADS 3 lesions according to clinically significant prostate cancer risk. Prostate. 2023;83:430–9.
Prive BM, Israel B, Janssen MJR, van der Leest MMG, de Rooij M, van Ipenburg JA, et al. Multiparametric MRI and (18)F-PSMA-1007 PET/CT for the detection of clinically significant prostate cancer. Radiology. 2024;311:e231879.
Chen M, Zhang Q, Zhang C, Zhao X, Marra G, Gao J, et al. Combination of (68)Ga-PSMA PET/CT and Multiparametric MRI improves the detection of clinically significant prostate cancer: a lesion-by-lesion analysis. J Nucl Med. 2019;60:944–9.
Wondergem M, van der Zant FM, Broos WAM, Knol RJJ. Matched-Pair Comparison of (18)F-DCFPyL PET/CT and (18)F-PSMA-1007 PET/CT in 240 prostate cancer patients: interreader agreement and lesion detection rate of suspected lesions. J Nucl Med. 2021;62:1422–9.
Funding
This work was supported by the Natural Science Basic Research Programme of Shaanxi Province (No.2020JZ-38); and the New Medical and Technology of the First Affiliated Hospital of Xi’ an Jiaotong University (No.XJYFY-2019J1).
Author information
Authors and Affiliations
Contributions
X.D. and H.X. contributed to the conceptualisation; L.L., R.W., X.W., R.C., W.D., Y.L., and H.L. contributed to the acquisition of data; L.L., L.B., J.S., Y.L., and H.L. contributed to the analysis of data; L.L., R.W., X.W., L.B., J.S., R.C., Y.L., and Y.L. contributed to the writing of the original draft and visualisation. X.D., H.L., and H.X. edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Affiliated Hospital of Xi’ an Jiaotong University (No. KYLLSL-2023-248). Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 2, 3, and 4.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, L., Wang, R., Bai, L. et al. The accuracy of fluorine 18-labelled prostate-specific membrane antigen PET/CT and MRI for diagnosis of prostate cancer in PSA grey zone. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02934-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02934-x