Abstract
Mosquito-borne diseases, such as malaria, dengue, and Zika, pose major public health challenges globally, affecting millions of people. The growing resistance of mosquito populations to synthetic insecticides underscores the critical need for effective and environmentally friendly larvicides. Although chemical pesticides can initially be effective, they often lead to negative environmental consequences and health hazards for non-_target species, including humans. This study aimed to evaluate the larvicidal effects of Trachyspermum ammi essential oil and Delphinium speciosum extract on the larvae of three major mosquito species: Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus. Mosquito larvae of Ae. aegypti, An. stephensi, and Cx. quinquefasciatus were reared under controlled laboratory conditions. The larvicidal activity of T. ammi essential oil and D. speciosum extract was evaluated through standard bioassays, using various concentrations of essential oils (10, 20, 40, 80, and 160 ppm) and extracts (160, 320, 640, 1280, and 2560 ppm) to determine the lethal concentration (LC50) values after 24 h of exposure. Fresh plant materials were collected, with the essential oil extracted via hydro-distillation, and the extract prepared using methanol solvent extraction. The chemical composition of T. ammi essential oil was examined using gas chromatography-mass spectrometry (GC-MS). Additionally, the preliminary analysis of the chemical compounds in D. speciosum extract was carried out using thin layer chromatography (TLC) and nuclear magnetic resonance spectroscopy (NMR) techniques. The results indicated that the essential oil of T. ammi exhibited more effective larvicidal activity compared to the D. speciosum extract. Specifically, the essential oil demonstrated LC50 values of 18 ppm for Cx. quinquefasciatus and 19 ppm for Ae. aegypti. In contrast, the D. speciosum extract showed the strongest larvicidal effect against An. stephensi, with an LC50 of 517 ppm. Concentrations of 40 ppm of the essential oil and 1280 ppm of the extract resulted in 100% mortality across all three species. Both the essential oil of T. ammi and the D. speciosum extract exhibited concentration-dependent larvicidal activity, and these results were statistically significant (p < 0.001) compared to the no-treatment group. GC-MS analysis revealed thymol (88.95%), o-cymen-5-ol (4.11%), and γ-terpinene (2.10%) as the major constituents of the T. ammi essential oil. Additionally, TLC verified the presence of alkaloids in both chloroform and methanolic extracts. Proton NMR identified a diterpene structure for these alkaloids. These findings suggest that T. ammi essential oil is a promising candidate for natural mosquito control strategies. Given its efficacy, further research is warranted to explore its potential in integrated vector management programs.
Similar content being viewed by others
Introduction
Mosquitoes (Diptera: Culicidae) are responsible for spreading important human parasites and microbes1, leading to major diseases and death, which impose a substantial economic burden globally2. Mosquitoes pose significant public health risks by transmitting diseases such as lymphatic filariasis, malaria, dengue fever, yellow fever, and Zika virus3. This emphasizes the crucial role of mosquito vector control in tropical and subtropical regions worldwide4,5,6.
Cx. quinquefasciatus plays a crucial role as a vector for both lymphatic filariasis and West Nile virus (WNV)7. Lymphatic filariasis, commonly referred to as elephantiasis, is recognized as one of the most significant neglected infectious diseases8 and the second most common global mosquito-borne disease9. Over 882 million people in 44 countries worldwide remain threatened by lymphatic filariasis10.
Malaria is primarily transmitted to humans through the bites of infected female Anopheles stephensi11. In 2022, approximately 249 million cases of malaria were reported worldwide, resulting in 608000 malaria-related deaths across 85 countries12.
Dengue fever is a mosquito-borne viral infection that spreads to humans through the bites of infected mosquitoes, primarily Aedes aegypti mosquito13. Dengue has become a major global health issue, affecting approximately four billion people across 130 countries14. Annually, approximately 100–400 million infections occur, putting a significant portion of the world’s population at risk14.
While the treatment of these diseases is challenging, prevention offers a viable and effective strategy to reduce the burden of these disease and address the economic, emotional, and health consequences. Various approaches have been developed to manage vector populations and curb disease transmission in regions where these diseases are endemic15. Over the past several decades, synthetic insecticides have been employed to manage vectors and break the transmission cycle of vector-borne diseases16. However, the extensive use of synthetic pesticides to control mosquitoes presents significant risks, including toxicity to non-_target organisms and potential harm to environmental and human health17,18. Additionally, the use of these synthetic chemicals contributes to the development of resistance in mosquito populations19.
Recent strategies to control mosquito vectors involve eco-friendly approaches, including the use of botanical insecticides. It is essential to explore environmentally friendly alternatives in botanicals, such as plant extracts and essential oils (EO)20. EOs are volatile compounds present in various plant families, including Asteraceae, Rutaceae, Myrtaceae, Lauraceae, Lamiaceae, Apiaceae, Piperaceae, Poaceae, Zingiberaceae, and Cupressaceae21. These natural insecticides are specifically toxic to mosquito pests while being environmentally beneficial. It is well-known that plant metabolites are toxic to mosquitoes and can effectively manage their populations22.
Botanical components possess antifungal, antibacterial, antileishmanial, antimalarial, and insecticidal properties23,24,25,26. Natural substances, such as EO and plant extracts, exhibit a wide range of biological effects, including insecticidal, acaricidal, repellent, antifeedant, ovipositional deterrent, and growth inhibiting properties against insect pests, including mosquitos27,28,29,30. EOs represent a substantial source of biologically active monoterpenes and have been extensively documented for their bioactivities against insect pests. Several EOs and extracts with notable potential for mosquito control originate from the plant genera Tagetes spp.31, Mentha spp.32, Citrus spp.33, Trachyspermum spp.34, and Delphinium spp.35. The larvicidal potency of extract from Thymus plant36, Satureja species37, Pelargonium roseum38, Carum copticum39, Citrus aurantifolia40 against Cx. quinquefasciatus, An. stephensi, Ae, aegypti have been reported. Also, EOs derived from cassia, camphor, wintergreen, pine, and eucalyptus are already being utilized in various commercial products intended for mosquito control41.
Trachyspermum ammi is taxonomically classified under the Apiaceae family, which comprises approximately 347 genera and 12,816 species. The plant is widely distributed and cultivated in various regions, including Iran, Pakistan, Afghanistan, and India. The grayish-brown seeds (fruits) of T. ammi are commonly used for biomedical and nutritional proposes. The EO extracted from the seeds is a volatile oil possessing distinctive organoleptic and physicochemical characteristics42,43.
The Delphinium genus (Ranunculaceae) encompasses around 300 species globally, with 28 species found in Iran, including 9 species endemics to the region44. D. speciosum, a perennial herb found in Northwestern region of Iran, features villous stems that can grow up to 80 cm in height. These stems may be either simple or branched and are supported by woody roots. The leaves of this plant are rounded-pentagonal in shape, measuring between 3–12 mm in width, and are covered with villous hairs on both surfaces44. For many years, plants from the Delphinium genus have been traditionally used as herbal remedies, including insecticides, larvicides, antibacterial, and lice treatment35,45,46,47.
Despite the limited knowledge of the larvicidal properties of T. ammi and D. speciosum endemic to Iran, our study represents the first investigation into their effects against C. quinquefasciatus, An. stephensi, and Ae. aegypti. We also examined their chemical profiles, which could potentially be utilized for future biopesticide development.
Materials & methods
Mosquitoes rearing
The mosquito larvae employed in larvicidal tests including Ae. aegypti, An. stephensi, and Cx. quinquefasciatus, were sourced from a laboratory colony at the Department of Biology and Control of Disease Vectors, Faculty of Health, Hormozgan University of Medical Sciences in Bandar Abbas, Iran. These colonies were consistently maintained at 27 ± 1 °C, with a 12:12 light and dark photoperiod, and 65 ± 5% relative humidity. The larvae were fed daily with powdered fish food until pupation, ensuring a continuous supply for mosquito larvicidal experiments.
Collection of plants material
The D. speciosum plant which was collected in May 2023 from the Sahand Mountain in the Sprakhon region of East Azarbaijan Province (37 °82′N, 46 °40′E; altitude of 2480 m), and a voucher specimen (TBZMED 5004) was deposited in the Herbarium of the Faculty of Pharmacy, Tabriz University of Medical Sciences. The T. ammi seeds were procured from Shafa Pajohan Sabz, a knowledge-based firm located in Tabriz, Iran. The collected plants were identified by Dr. Atefeh Ebrahimi, a botanist at the Herbarium of the Faculty of Pharmacy, Tabriz University of Medical Sciences. Harvesting of the plants was conducted in accordance with the regulations issued by the Natural Resources & Watershed Management Organization-I.R. of Iran and the Research Institute of Forests and Rangelands. According to these regulations, the harvesting of Delphinium plants for laboratory purposes is permitted without restrictions.
Plant extract preparation
Following the collection and washing of D. speciosum, the plant was subjected to a drying process under laboratory conditions. The aerial parts of the plant were then pulverized and subjected to extraction via the Soxhlet method, utilizing petroleum ether, chloroform, and methanol sequentially. A portion of the resulting crude methanolic extract, weighing 5 g, was subsequently employed for bioassay analysis. Different concentrations (160, 320, 640, 1280, and 2560 ppm) were derived from the stock solution48,49.
Essential oil extraction
The T. ammi seeds were pulverized, and their essential oil was extracted using hydro-distillation with a Clevenger apparatus over a period of 3 h. The essential oil was subsequently dried using anhydrous sodium sulphate and preserved at a temperature of −4 °C for use in larvicidal bioassay tests50.
Larvicidal bioassay
Bioassay tests were conducted in accordance with the World Health Organization’s standard method51. Total T. ammi essential oil and D. speciosum extract were initially dissolved in 99% ethanol and 99% methanol, respectively, as co-solvents. The solutions were stirred for 30 s with a glass rod. For the larval test, 25 larvae at the late 3rd and early 4th instars of Ae. aegypti, An. stephensi, and Cx. quinquefasciatus were collected using a fine mesh strainer and then gently transferred by tapping into four 400-mL glass beakers containing 249 mL of dechlorinated tap water. Each concentration was replicated at least four times, comprising a total of 100 larvae. The test medium was prepared by combining 1 mL of the appropriate dilution of essential oil or total extract in co-solvents with 249 mL of water. Controls included batches of mosquitoes from the colony exposed to water and the solvents alone. The larvae were exposed to different concentrations of 10, 20, 40, 80, and 160 ppm of essential oil, and 160, 320, 640, 1280, and 2560 ppm of total extract in dechlorinated tap water for 24 h, with no provision of food during this period.
Gas chromatography/Mass spectroscopy analysis
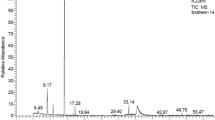
The composition of T. ammi EO was analyzed using a chromatography-mass spectrometry instrument (Shimadzu, QP-5050 A) equipped with a DB-1 capillary column (60 m length, 0.25 mm inner diameter, and 0.25 μm film thickness). An electron impact ionization (EI) system applying 70 eV ionization energy was used for the identification of the essential oil’s volatile components. Helium was employed as the carrier gas at a flow rate of 1 mL/min, operating in constant linear velocity mode. The injector and detector temperatures were set at 250 °C. The oven temperature was held at 50 °C for 3 min and then was programed from 50 to 265 °C at the rate of 2.5 °C/min, and was kept at this temperature for 6 min. After dilution of the essential oil in n-hexane (1:100), 1 µL of essential oil solution was injected manually.
Identification of each volatile component was carried out using calculated retention indices (RI) based on n-alkanes chromatogram (C8–C24) under the same gas chromatography condition. The EO constituents were identified by comparing their RI values and mass spectra with those in several Mass Spectral libraries such as Wiley and NIST. The results are presented as the area of Mass response in relative percentages52.
NMR analysis of extracts
Since Delphinium species are mainly known for having diterpene alkaloids, and most published sources consider these compounds responsible for the anti-parasitic effects of these plants, so it was tried to check the presence or absence of alkaloid compounds in the used extracts. A preliminary study was conducted to determine the alkaloid content of the extracts using TLC on precoated-silica gel plate (TLC Silica gel 60 F254, Merck). Additionally, NMR spectroscopy was used to determine the nature of alkaloids in the extracts. It should be noted that the NMR guided isolation method is a valuable technique that has been widely used by researchers in metabolomic studies and drug discovery. This very powerful tool provides the ability to detect different categories of chemical compounds in the extract at the same time53,54,55,56,57. NMR experiments were recorded with a Bruker UltraShield-400 spectrometer (Bruker, Germany) in deuterated chloroform and TMS used as an internal standard.
Statistical analysis
In the current study, the independent variables include different larval species and treatment groups exposed to various concentrations of essential oils and plant extracts, while the dependent variable is the mosquito mortality rate. All experiments were conducted in quadruplicate, data are expressed as mean ± standard deviation. We assessed the normality of the dependent variable using graphical methods and statistical tests. Due to the large sample size, the central limit theorem supports the assumption of normality in the data58. Lethal concentrations (LC50 and LC90), along with 95% confidence intervals and probit equations, were determined through probit regression analysis. A Three-Way ANOVA followed by Tukey’s HSD test (95% confidence interval, SPSS v.25) was used to compare larvicidal activity. Graphs were generated using GraphPad Prism v.8. Statistical significance was defined as p < 0.05. The effect size (ES) was calculated using Cohen's d, which is defined as the difference between two means divided by the pooled standard deviation. Cohen's guidelines for interpreting effect size are: small effect: d≈0.2, Medium effect: d≈0.5, Large effect: d≈0.8. This quantification provided an objective measure of the practical significance of the findings59.
Results
Larvicidal effect of samples
In this study, we examined the larvicidal effect of T. ammi essential oil at different concentrations ranging from 10 to 160 ppm and D. speciosum extract at various concentrations between 160 and 2560 ppm on three mosquito species: An. stephensi, Cx. quinquefasciatus and Ae. aegypti. The findings are summarized in (Table 1).
As shown in Table 1, The An. stephensi demonstrated the highest larval mortality rate when treated with D. speciosum extract, reaching 100% mortality at a concentration of 1280 ppm. For Cx. quinquefasciatus and Ae. aegypti larvae treated with this extract, mortality rates at the highest concentration of 2560 ppm were 98 and 99%, respectively. The lowest mortality rate was observed in Cx. quinquefasciatus larvae treated with D. speciosum extract, showing only 5% mortality at a concentration of 160 ppm. For larvae treated with T. ammi EO, the highest mortality rates were observed in Cx. quinquefasciatus and Ae. aegypti, with mortality rates of 96% and 94%, respectively, at a concentration of 40 ppm. In contrast, the mortality rate for An. stephensi larvae treated with 40 ppm of T. ammi EO was reported to be 45%. The lowest larval mortality rates at 10 ppm of T. ammi EO were recorded as 6% for An. stephensi, 13% for Cx. quinquefasciatus, and 11% for Ae. aegypti.
A clear dose-dependent relationship was observed between the concentration of T. ammi EO and D. speciosum extract and the mortality rates of mosquito larvae. As the concentration of T. ammi EO increased, the mortality rates among An. stephensi, Cx. quinquefasciatus, and Ae. aegypti also increased significantly. Specifically, at lower concentrations (10 and 20 ppm), mortality rates ranged from 13 to 54%, while at higher concentrations (160 ppm), mortality rates reached up to 99% for An. stephensi and 100% for Cx. quinquefasciatus and Ae. aegypti. Similarly, D. speciosum extract exhibited a dose-dependent effect, with mortality rates increasing from 10, 5, and 6% at 160 ppm to 100% at 2560 ppm for An. stephensi, Cx. quinquefasciatus, and Ae. aegypti. Statistical analysis confirmed a significant dose-dependent relationship (p < 0.05) between the concentration of T. ammi EO and D. speciosum extract and larval mortality, indicating that both are effective larvicides, with higher concentrations leading to increased mortality in mosquito populations (Table 2).
The results of this study also revealed that the average mortality rates differed significantly among the various larval species (p < 0.02). The ANOVA test indicated statistically significant differences in average mortality rates between An. stephensi and Cx. quinquefasciatus (p = 0.03), as well as between An. stephensi and Ae. aegypt (p = 0.04). However, the difference in average mortality rates between Cx. quinquefasciatus and Ae. aegypti was not statistically significant (p = 0.06( (Table 3).
A significant difference in the average larval mortality percentage was observed among the treatment groups (p = 0.001). The post-hoc ANOVA tests revealed a significant difference between the groups treated with T. ammi EO and D. speciosum extract (p = 0.02). Additionally, the average mortality percentage in the groups treated with either T. ammi EO or D. speciosum extract was significantly higher compared to the untreated group (p < 0.001) (Table 3).
The average mortality percentage varied across groups with different therapeutic concentrations, and this variation was statistically significant (p = 0.001). In the T. ammi EO treatment group, concentrations ranging from 20 to 160 ppm showed a significant difference compared to the untreated group (p < 0.001), while the 10 ppm concentration did not exhibit a statistically significant difference (p = 1.00).
In the group treated with D. speciosum extract, concentrations between 640 and 2560 ppm exhibited a significant difference compared to the untreated group (p < 0.001), while the 10 ppm concentration did not show a significant difference from the untreated group (p = 0.98) (Table 3).
The comparison of larval mortality percentages at different concentrations in the two treatment groups, T. ammi and D. speciosum, indicated no statistically significant difference between the highest concentration of D. speciosum (2560 ppm) and the concentrations of 40, 80, and 160 ppm of T. ammi (p > 0.05). Additionally, the lowest concentration of T. ammi (10 ppm) did not show a statistically significant difference compared to the concentrations of 160 to 640 ppm of D. speciosum (p > 0.05). However, the mortality percentages at other concentrations in the two treatment groups differred significantly (p < 0.05).
The mean difference (MD) and standardized mean difference (SMD) values, as well as the interpretation areas of the effect size, have been calculated and presented in (Tables 4, 5). The effect size interpretation showed that concentrations of 1280 and 2560 ppm of D. speciosum were more effective in increasing larval mortality rate compared to other concentrations tested (Table 4). Additionally, the concentrations of 40, 80 and 160 ppm of T. ammi were identified as highly effective (Table 5).
According to the dose-response curve shown in (Fig. 1), the LC50 value for the essential oil derived from T. ammi was calculated as 48.7, 18.3, and 19.7 ppm for An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively. The LC50 value for the extract of D. speciosum was determined as 517, 991.5, and 887.4 ppm for An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively.
Probit regression analysis of larvicidal data, shown in (Table 6), determined the LC50 and LC90 values for D. speciosum’s extract and EO of T. ammi. The LC90 value for the EO was calculated as 143.3, 35.8, and 40.2 ppm for An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively. The LC90 value for the extract was determined as 1157, 2662, and 2530 ppm for An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively.
Essential oil composition
Chemical compounds of the EO of T. ammi are presented in (Table 7). The volatile oil was characterized by the presence of 6 constituents, representing 98.09% of the total compounds. Thymol (88.95%), o-cymen-5-ol (4.11%), and γ‑terpinene (2.10%) were identified as the most abundant phytochemicals. All of the compounds belong to monoterpenes. Oxygenated monoterpenes are the main class of compounds in EO composition. The remaining compounds belong to hydrocarbon monoterpenes (Table 7).
Extract composition
TLC method was used to confirm the presence of alkaloids. Spraying the dragendroff reagent on the TLC surface revealed the orange spots of alkaloids (Fig. 2). The resulting chromatogram showed that alkaloids are present only in the chloroform and methanolic extracts, and the petroleum ether extract does not contain these compounds. Examination of proton NMR spectra showed that the alkaloids in the chloroform and methanol extracts have a diterpene structure (Fig. 3).
Discussion
The development of eco-friendly and safe insecticides derived from natural sources, such as plants, is vital due to their precise _targeting abilities and reduced risk of bioaccumulation. Utilizing natural control methods, like biopesticides, offers an effective strategy to combat insecticide resistance in mosquitoes60,61. In this study, we evaluated the efficacy of T. ammi essential oil and D. speciosum extract as larvicides against mosquito vectors of malaria, dengue fever, and filariasis.
Our study demonstrated the strong larvicidal effects of D. speciosum extract and T. ammi EO against three primary mosquito vectors: An. stephensi, Cx quinquefasciatus, and Ae. aegypti. These results are consistent with previous studies that investigated the toxic activity of Delphinium cardiopetalum extract against agricultural pest larvae62. Similar studies have demonstrated that extracts from Delphinium cultorum exhibit strong larvicidal effects on Ae. aegypti larvae63. The current study found mortality ratees ranging from 6 to 99% in Ae. aegypti, 10 to 100% in An. stephensi, and 5 to 98% in Cx. quinquefasciatus larvae treated with D. speciosum extracts at concentrations of 160 to 2560 ppm. These findings align with the 30–90% mortality observed in studies that reported significant larvicidal activity of 31 Delphinium species extracts against mosquito larvae64. Also, the findings of this study are consistent with previous research that reported significant larvicidal effects of Delphinium staphisagria against Leishmania spp, with a 100% mortality rate at 400 ppm65, which is higher than the 30–87% mortality rate at 200–1600 ppm observed in Ae. aegypti35,66.
In contrast to previous studies that indicated minimal insecticidal activity of Delphinium staphisagria against Pediculus species67, our study revealed a strong efficacy of D. speciosum extract against Ae. Aegypti, achieving a mortality rate of 99% at 2000 ppm. Our findings are also consistent with studies showing that the green synthesis of silver nanoparticles using Delphinium denudatum root extract possesses both antibacterial and mosquito larvicidal properties, with reported mortality rates of 95% at 4000 ppm in mosquito larvae47.
Many other studies have also shown that extracts from different plants have strong larvicidal effects on An. stephensi and Cx. quinquefasciatus, with reported mortality percentages ranging from 5 to 90%, which closely align with the results of our study20,68,69,70,71,72.
The results of previous studies showed that the EO of T. ammi seeds has moderate larvicidal effects on small hive beetles (Aethina tumida), with a mortality rate of 36.25% at the highest concentration (48 ppm). In contrast, the results of our study shows that the mortality rate of the important vector species at a concentration of 40 ppm was 96%73. T. ammi exhibited high potency against larvae in this study, with a similar effect recorded against Cx. quinquefasciatus, Ae. aegypti, and An. stephensi, achieving mortality rates of 90, 97, 100%, respectively41,43,74. While only a few studies have explored the larvicidal effects of D. speciosum extract and T. ammi EO, many studies have focused on the larvicidal properties of EOs from other plants on important vector species. A similar finding has been recorded for Cx. quinquefasciatus, Ae. aegypti, and An. stephensi20,71,75,
Consistent with our results, several past studies have reported statistically significant variations in the susceptibility of different mosquito larvae to plant-derived larvicides33,76,77,78. However, in contrast to our findings, a few previous research have reported no statistically significant differences in the larvicidal efficacy of plant extracts across different mosquito species79,80.
The statistically significant differences in the average larval mortality can be attributed to several factors. First, there is a variation in larval physiology and biochemistry, meaning different larval species may have different physiological and biochemical responses to the same treatment. Second, there is a differential penetration or mode of action of the larvicides, which means that the effectiveness of the larvicides can vary depending on how they penetrate the larvae or their mode of action81,82. These statistically significant differences in the average larval mortality underscore the importance of considering species-specific responses when developing and deploying plant-derived larvicides.
The results of our study indicated that T. ammi EO demonstrated stronger larvicidal activity than D. speciosum extract against all tested species. This increased effectiveness is attributed to its higher concentration of bioactive compounds. These EOs disrupt larval physiological processes and possess antimicrobial properties, which may enhance their effectiveness72. This finding aligns with previous studies, further confirming the superior efficacy of essential oil83,84. EO with an LC50 value less than 50 ppm are deemed very active, those between 50 and 100 ppm as active, and those over 100 ppm are considered weak or inactive85. For extracts, toxicity varies with LC50 values: under 100 ppm is highly toxic, 100–500 ppm is moderately toxic, 500–1000 ppm is low toxicity, and above 1000 ppm is non-toxic86. Based on this classification, the LC50 of T. ammi EO and D. speciosum extract from our study categorizes them as highly toxic and moderately toxic against all three vector species, respectively.
Our findings revealed that both plant-derived larvicides exhibited significant, dose-dependent larvicidal activities across all tested mosquito species, which are in line with the results of previous studies33,72,78,83. The LC50 of the T. ammi EO against An. stephensi larvae was 49 ppm, which is lower than the LC50 (81 ppm) reported for the same EOs in a previous study41. Similarly, the LC50 values of the EO from T. ammi against Ceratitis capitata, Tribolium castaneum, and Aethina tumidawas were reported to be 46, 15, and 52 ppm, respectively34,73,87, and the LC50 of the D. speciosum extract against Aedes larvae was 887 ppm, which is notably higher than the LC50 (244 ppm) documented for the same crude extract in another investigation35.
The effect size analysis of this study revealed that D. speciosum extract exhibited potent effects solely at concentrations of 1280 and 2560 ppm. In contrast, T. ammi essential oil demonstrated effects ranging from moderate to strong at concentrations between 20 and 160 ppm. Moreover, based on our knowledge, there were no reports found on the effect size of the larvicidal impact of D. speciosum extract and T. ammi EO against An. stephensi, Cx. quinquefasciatus, and Ae. aegypti. The findings on effect size and effective concentration were highly beneficial for enhancing larval mortality rates and applying them in real-world scenarios. In this research, the practical concentrations used were 1280 ppm for D. speciosum and 40 ppm for T. ammi.
Previous studies reported that γ‑terpinene, carvacrol, p-cymene, and thymol were the main phytochemicals in the EO of T. ammi52,88,89,90,91,92,93,94,95. These studies investigated different chemotypes of T. ammi from different localities using various extraction methods. Our findings on the chemical constituents of the plant are consistent with previous findings. The minor differences in the chemical compositions reported by different studies may be associated with variable climate, geographical conditions, genetics, and extraction techniques. Previous studies show that most of the alkaloids in the genus Delphinium have diterpene structures96. As shown in our results, several alkaloid compounds were observed in chloroform and methanolic extracts.
The mechanisms of action of essential oils and total plant extracts against mosquito larvae are multifaceted and distinct. Essential oils primarily exert their larvicidal effects through contact toxicity, neurotoxicity, and enzyme inhibition. Upon contact, essential oils disrupt the larvae’s cuticle, leading to desiccation and death. Neurotoxic components, such as monoterpenes and sesquiterpenes, interfere with the nervous system, causing paralysis and mortality. Additionally, some essential oils inhibit acetylcholinesterase, an enzyme crucial for nerve signal transmission, further disrupting larval neural functions. In contrast, total plant extracts, which encompass a broader range of bioactive compounds, act through multiple mechanisms including growth disruption, metabolic inhibition, and oviposition deterrence. The diverse chemical constituents in plant extracts, such as alkaloids, flavonoids, and saponins, interfere with larval development and molting processes, disrupt energy metabolism, and deter female mosquitoes from laying eggs in treated water. These combined actions make both essential oils and plant extracts effective larvicides, though their specific mechanisms and applications may vary depending on the plant species and extraction methods used97,98,99,100.
Botanically derived natural larvicides are gaining attention as safety and environmentally friendly alternatives to synthetic insecticides for mosquito control. Despite their benefits, such as lower chemical residues and reduced toxicity to non-_target organisms, there are still potential environmental impacts and safety concerns that must be considered. Additionally, the persistence and degradation of these natural compounds in the environment are not always well understood. While the safety profile of natural larvicides for humans and animals is generally better than that of synthetic insecticides, some natural compounds can still pose risks. Furthermore, the variability in the composition of natural extracts can lead to inconsistent efficacy and safety profiles. Standardization and rigorous testing are essential to ensure the safe use of these products101,102,103.
Our study offers encouraging indications of the larvicidal potential of D. speciosum extract and T. ammi EO. However, it’s crucial to recognize some limitations. The experiments were carried out in a controlled laboratory environment. To evaluate the effectiveness and practicality of these plant-derived compounds in real-world vector control programs, additional field-based assessments are required. Furthermore, identifying and characterizing the specific phytochemicals that contribute to the observed larvicidal activities calls for more in-depth investigation.
Conclusion
Our study has highlighted the substantial larvicidal effects of D. speciosum extract and T. ammi EO against three primary disease vector mosquitoes. The larvicidal activity of T. ammi essential oil surpasses that of D. speciosum extract, with the optimal concentration being 40 ppm. Additionally, among the species studied, Cx. quinquefasciatus exhibits the highest sensitivity to these plant-derived treatments. Thymol is the main component of T. ammi EO, and the alkaloids in D. speciosum extract have diterpene structures. Considering the effect size values, along with the dose-dependent trends and species-specific responses can help researchers gain a more comprehensive understanding of the real-world implications and potential utility of the plant-derived larvicides investigated in this study. Future studies should include both laboratory and field trials to evaluate their effectiveness against diverse mosquito populations, assess its environmental impact, and determine optimal application methods for their practical use in vector control.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Reinhold, J. M., Lazzari, C. R. & Lahondère, C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects 9, 158 (2018).
Gabrieli, P., Smidler, A. & Catteruccia, F. Engineering the control of mosquito-borne infectious diseases. Genome Biol. 15, 1–9 (2014).
CDC. Centers for Disease Control and Prevention. Where mosquitoes live 2024. https://www.cdc.gov/mosquitoes/about/where-mosquitoes-live_1.html. (accessed 22 June 2024).
Wilder-Smith, A. et al. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 17, e101–e106 (2017).
Benelli, G. & Mehlhorn, H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol. Res. 115, 1747–1754 (2016).
Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 114, 2801–2805 (2015).
Samy, A. M. et al. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS ONE 11, e0163863 (2016).
Benelli, G. et al. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 66, 166–171 (2017).
Thandapani, K. et al. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO 2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 25, 10328–10339 (2018).
WHO. World Health Organisation. lymphatic philariasis key facts 2023 https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis. (accessed 1 June 2023).
Liu, Q. et al. Possible potential spread of Anopheles stephensi, the Asian malaria vector. BMC Infect. Dis. 24, 333 (2024).
WHO. World Health Organisation. Malaria key facts 2023 https://www.who.int/news-room/fact-sheets/detail/malaria. (accessed 4 December 2023).
Brady, O. J. et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 6, e1760 (2012).
WHO. World Health Organisation. Dengue and severe dengue https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. (accessed 17 March 2023).
Laith, A. E., Alnimri, M., Ali, H., Alkhawaldeh, M. & Mihyar, A. Mosquito-borne diseases: assessing risk and strategies to control their spread in the Middle East. J. Biosaf. Biosecur. 6, 1–12 (2024).
Floore, T. G. Mosquito larval control practices: Past and present. J. Am. Mosq. Control Assoc. 22, 527–533 (2006).
Naqqash, M. N., Gökçe, A., Bakhsh, A. & Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 115, 1363–1373 (2016).
Mansouri, A. et al. The environmental issues of DDT pollution and bioremediation: A multidisciplinary review. Appl. Biochem. Biotechnol. 181, 309–339 (2017).
Serrão, J. E., Plata-Rueda, A., Martínez, L. C. & Zanuncio, J. C. Side-effects of pesticides on non-_target insects in agriculture: A mini-review. Sci. Nat. 109, 17 (2022).
Selvakumaran, J. et al. Evaluation of mosquitocidal, histopathological and non-_target effect of botanical pesticides from Stemodia viscosa and their mixtures against immature stages of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. . Biologia 79, 1425–1437 (2024).
Khater, H. F. Prospects of botanical biopesticides in insect pest management. Pharmacologia 3, 641–656 (2012).
Govindarajan, M., Rajeswary, M. & Benelli, G. Chemical composition, toxicity and non-_target effects of Pinus kesiya essential oil: An eco-friendly and novel larvicide against malaria, dengue and lymphatic filariasis mosquito vectors. Ecotoxicol. Environ. Saf. 129, 85–90 (2016).
Nenaah, G. E. & Ibrahim, S. I. Chemical composition and the insecticidal activity of certain plants applied as powders and essential oils against two stored-products coleopteran beetles. J. Pest Sci. 84, 393–402 (2011).
Sousa, Z. L. et al. Biological activities of extracts from Chenopodium ambrosioides Lineu and Kielmeyera neglecta Saddi. Ann. Clin. Microbiol. Antimicrob. 11, 1–7 (2012).
Pavela, R. et al. Clausena anisata and Dysphania ambrosioides essential oils: From ethno-medicine to modern uses as effective insecticides. Environ. Sci. Pollut. Res. 25, 10493–10503 (2018).
Kavallieratos, N. G. et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium everts. Food Chem. Toxicol. 139, 111255 (2020).
Haddi, K. et al. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 76, 2286–2293 (2020).
Dai, D. N. et al. Chemical compositions, mosquito larvicidal and antimicrobial activities of essential oils from five species of cinnamomum growing wild in north central Vietnam. Molecules 25, 1303 (2020).
Eden, W. T., Alighiri, D., Supardi, K. I. & Cahyono, E. The mosquito repellent activity of the active component of air freshener gel from java citronella oil (Cymbopogon winterianus). J. Parasitol. Res. 202, 1–5 (2020).
Silvério, M. R. S., Espindola, L. S., Lopes, N. P. & Vieira, P. C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 25, 3484 (2020).
Vasudevan, P., Kashyap, S. & Sharma, S. Tagetes: A multipurpose plant. Bioresour. Technol. 62, 29–35 (1997).
Ansari, M., Vasudevan, P., Tandon, M. & Razdan, R. Larvicidal and mosquito repellent action of peppermint (Mentha piperita) oil. Bioresour. Technol. 71, 267–271 (2000).
Sanei-Dehkordi, A., Moemenbellah-Fard, M. D., Saffari, M., Zarenezhad, E. & Osanloo, M. Nanoliposomes containing limonene and limonene-rich essential oils as novel larvicides against malaria and filariasis mosquito vectors. BMC Complement. Med. Ther. 22, 140 (2022).
Benelli, G. et al. Carlina acaulis and Trachyspermum ammi essential oils formulated in protein baits are highly toxic and reduce aggressiveness in the medfly Ceratitis capitata. Ind. Crops Prod. 161, 113191 (2021).
Sen-Utsukarci, B. et al. The cytotoxicity and insecticidal activity of extracts from Delphinium formosum boiss. & huet. Istanb. J. Pharm. 49, 148–153 (2019).
Pitarokili, D., Michaelakis, A., Koliopoulos, G., Giatropoulos, A. & Tzakou, O. Chemical composition, larvicidal evaluation, and adult repellency of endemic Greek Thymus essential oils against the mosquito vector of West Nile virus. Parasitol. Res. 109, 425–430 (2011).
Michaelakis, A., Theotokatos, S. A., Koliopoulos, G. & Chorianopoulos, N. G. Essential oils of Satureja species: insecticidal effect on Culex pipiens larvae (Diptera: Culicidae). Molecules 12, 2567–2578 (2007).
Tabari, M. A., Youssefi, M. R., Esfandiari, A. & Benelli, G. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil against the West Nile and filariasis vector Culex pipiens (Diptera: Culicidae). Res. Vet. Sci. 114, 36–40 (2017).
Al-Mekhlafi, F. A. Larvicidal, ovicidal activities and histopathological alterations induced by Carum copticum (Apiaceae) extract against Culex pipiens (Diptera: Culicidae). Saudi J. Biol. Sci. 25, 52–56 (2018).
Andrade-Ochoa, S. et al. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): synergism–antagonism effects. Insects 9, 25 (2018).
Pandey, S., Upadhyay, S. & Tripathi, A. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) sprague seeds against Anopheles stephensi. Parasitol. Res. 105, 507–512 (2009).
Kadam, S. S., More, S. B. & Waghmare, J. S. Tranchyspermum Ammi: Natural pesticides. J. Biopest. 10, 90–98 (2017).
Bhadra, P. An overview of Ajwain (Trachyspermum ammi). Indian J. Nat. Sci. 10, 18466–182474 (2020).
Gheybi, S., Asnaashari, S., Moghaddam, S. B., Ebrahimi, A. & Afshar, F. H. Volatile components of aerial parts of Delphinium speciosum MB growing in Iran. J. Rep. Pharm. Sci. 4, 191–195 (2015).
Vicentini, C. B., Manfredini, S. & Contini, C. Ancient treatment for lice: A source of suggestions for carriers of other infectious diseases?. Infez. Med. 26, 181–192 (2018).
Shan, L., Chen, L., Gao, F. & Zhou, X. Diterpenoid alkaloids from Delphinium naviculare var. lasiocarpum with their antifeedant activity on Spodoptera exigua. Nat. Prod. Res. 33, 3254–3259 (2019).
Suresh, G. et al. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 127, 61–66 (2014).
Dey, P. et al. Recent Advances in Natural Products Analysis (Elsevier, 2020).
Pereira, A. G. et al. Natural Secondary Metabolites: From Nature, Through Science, to Industry (Springer, 2023).
Kumar, A., Shukla, R., Singh, P. & Dubey, N. K. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food Chem. Toxicol. 48, 539–543 (2010).
WHO. World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides (No. WHO/CDS/WHOPES/GCDPP/2005.13).
Bahadori, M. B., Asnaashari, S. & Nazemiyeh, H. Fatty acid profile of roots and aerial parts of Ruscus hyrcanus Woronow. Pharma. Sci. 25, 78–81 (2019).
Wei, Y. et al. 1H NMR guided isolation of 3-arylisoquinoline alkaloids from Hypecoum erectum L. and their anti-inflammation activity. Phytochemistry 222, 114093 (2024).
Xiao, J. et al. 1H NMR-guided isolation of hasubanan alkaloids from the alkaloidal extract of Stephania longa. Bioorg. Chem. 139, 106717 (2023).
Zhao, C.-X. et al. 1H-NMR-guided isolation of enantiomeric coumarin-monoterpenes with anti-inflammatory activity from Gerbera piloselloides. Phytochemistry 203, 113346 (2022).
Wang, A. D. et al. Isolation and structure determination of new saponins from Pulsatilla cernua based on an NMR-guided method and their anti-proliferative activities. Phytochem. Lett. 27, 9–14 (2018).
Zhang, Y. et al. Isolation of a new monoterpenoid glycoside from anhua dark tea based on an NMR-guided method and its cytotoxic activity against MDA-MB-231 and SH-SY5Y cell lines. Nat. Prod. Res. 36, 2015–2020 (2022).
Elliott, A. C. & Woodward, W. A. Statistical Analysis Quick Reference Guidebook: with SPSS Examples (Sage, 2007).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, 2013).
Rahimi, S., Vatandoost, H., Abai, M. R., Raeisi, A. & Hanafi-Bojd, A. A. Status of resistant and knockdown of West Nile vector, Culex pipiens complex to different pesticides in Iran. J. Arthropod Borne Dis. 13, 284 (2019).
Rahimi, S. et al. Resistant status of Culex pipiens complex species to different imagicides in Tehran Iran. J.Vect. Borne Dis. 57, 47–51 (2020).
González-Coloma, A. et al. Antifeedant delphinium diterpenoid alkaloids. Structure—Activity relationships. J. Agric. Food Chem. 46, 286–290 (1998).
Miles, J. E., Ramsewak, R. S. & Nair, M. G. Antifeedant and mosquitocidal compounds from delphinium× cultorum Cv. magic fountains flowers. J. Agric. Food Chem. 48, 503–506 (2000).
Ulubelen, A. et al. Insect repellent activity of diterpenoid alkaloids. Phytother. Res. 15, 170–171 (2001).
Ramírez-Macías, I. et al. Leishmanicidal activity of nine novel flavonoids from Delphinium staphisagria. Sci. World J. 2012, 203646 (2012).
Yan, Y., Li, X., Wang, Z., Yang, X. & Yin, T. C 18-diterpenoid alkaloids in tribe Delphineae (Ranunculaceae): Phytochemistry, chemotaxonomy, and bioactivities. RSC Adv. 12, 395–405 (2022).
Vicentini, C. B., Manfredini, S. & Contini, C. Ancient treatment for lice: A source of suggestions for carriers of other infectious diseases?. Le Infez. Med. 26, 181–192 (2018).
Alghamdi, A. A. & Basher, N. S. Efficacy of leaves and flowers ethanol extracts of the invasive species Lantana camara Linn as a mosquito larvicidal. Int. J. Mosq. Res. 7, 43–47 (2020).
Fasasi, K., Olawoyin, M. O., Rufai, A. M. & Iwalewa, Z. O. Anales de Biología (Servicio de Publicaciones de la Universidad de Murcia, 2024).
Mandal, P. & Chandra, G. Casearia tomentosa fruit extracts exposed larvicidal activity and morphological alterations in Culex quinquefasciatus and Aedes albopictus under in vitro and semi field conditions. BMC Res. Notes 17, 6 (2024).
Pirmohammadi, M. et al. Influence of agro-climatic conditions on chemical compositions and repellency effect of Mentha longifolia plant against malaria vector Anopheles stephensi. Toxin Rev. 42, 115–121 (2023).
Baz, M. M., Selim, A., Radwan, I. T., Alkhaibari, A. M. & Khater, H. F. Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. Sci. Rep. 12, 4406 (2022).
Bisrat, D. & Jung, C. Insecticidal toxicities of three main constituents derived from Trachyspermum ammi (L.) Sprague ex turrill fruits against the small hive beetles, Aethina tumida murray. Molecules 25, 1100 (2020).
Seo, S. M., Park, H. M. & Park, I. K. Larvicidal activity of ajowan (Trachyspermum ammi) and Peru balsam (Myroxylon pereira) oils and blends of their constituents against mosquito, Aedes aegypti, acute toxicity on water flea, Daphnia magna, and aqueous residue. J. Agric. Food Chem. 60, 5909–5914 (2012).
Kusman, I. T. et al. The potentials of Ageratum conyzoides and other plants from Asteraceae as an antiplasmodial and insecticidal for malaria vector: An article review. Infect. Drug Resist. 16, 7109–7138 (2023).
Ammar, S. et al. Essential oils from three Algerian medicinal plants (Artemisia campestris, Pulicaria arabica, and Saccocalyx satureioides) as new botanical insecticides?. Environ. Sci. Pollut. Res. 27, 26594–26604 (2020).
Benelli, G. et al. Ascaridole-rich essential oil from marsh rosemary (Ledum palustre) growing in Poland exerts insecticidal activity on mosquitoes, moths and flies without serious effects on non-_target organisms and human cells. Food Chem. Toxicol. 138, 111184 (2020).
Žabka, M. et al. Antifungal and insecticidal potential of the essential oil from Ocimum sanctum L. against dangerous fungal and insect species and its safety for non-_target useful soil species Eisenia fetida (Savigny, 1826). Plants 10, 2180 (2021).
Huong, L. T. et al. Essential oils of Zingiber species from Vietnam: Chemical compositions and biological activities. Plants 9, 1269 (2020).
An, N. T. G. et al. Mosquito larvicidal activity, antimicrobial activity, and chemical compositions of essential oils from four species of Myrtaceae from central Vietnam. Plants 9, 544 (2020).
Inaba, K. et al. Molecular action of larvicidal flavonoids on ecdysteroidogenic glutathione S-transferase Noppera-bo in Aedes aegypti. BMC Biol. 20, 43 (2022).
Antonio-Nkondjio, C., Sandjo, N. N., Awono-Ambene, P. & Wondji, C. S. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: Key parameters for success. Parasites Vect. 11, 1–12 (2018).
García-Díaz, J. et al. Larvicidal and adulticidal activity of essential oils from four cuban plants against three mosquito vector species. Plants 12, 4009 (2023).
Chaves, R. D. S. B. et al. Evaluation of larvicidal potential against larvae of Aedes aegypti (Linnaeus, 1762) and of the antimicrobial activity of essential oil obtained from the leaves of Origanum majorana L.. PLoS ONE 15, e0235740 (2020).
Kiran, S. R., Bhavani, K., Devi, P. S., Rao, B. R. & Reddy, K. J. Composition and larvicidal activity of leaves and stem essential oils of Chloroxylon swietenia DC against Aedes aegypti and Anopheles stephensi. Bioresour. Technol. 97, 2481–2484 (2006).
Nguta, J. et al. Biological screening of Kenya medicinal plants using Artemia salina L. (Artemiidae). Pharmacologyonline 2, 458–478 (2011).
Chaubey, M. K. Insecticidal activity of Trachyspermum ammi (Umbelliferae), Anethum graveolens (Umbelliferae) and Nigella sativa (Ranunculaceae) essential oils against stored-product beetle Tribolium castaneum herbst (Coleoptera: Tenebrionidae). Afr. J. Agric. Res. 2, 596–600 (2007).
Sahaf, B. Z., Moharramipour, S. & Meshkatalsadat, M. H. Chemical constituents and fumigant toxicity of essential oil from Carum copticum against two stored product beetles. Insect Sci. 14, 213–218 (2007).
Moein, M. R. et al. Trachyspermum ammi (L.) sprague: Chemical composition of essential oil and antimicrobial activities of respective fractions. J. Evid. Based Complement. Altern. Med. 20, 50–56 (2015).
Rasooli, I. et al. Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int. J. Food Microbiol. 122, 135–139 (2008).
Khajeh, M., Yamini, Y., Sefidkon, F. & Bahramifar, N. Comparison of essential oil composition of Carum copticum obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 86, 587–591 (2004).
Srivastava, M., Baby, P. & Saxena, A. GC-MS investigation and antimicrobial activity of the essential oil of Carum copticum benth & hook. Acta Aliment. 28, 291–295 (1999).
Mohagheghzadeh, A., Faridi, P. & Ghasemi, Y. Carum copticum Benth & Hook essential oil chemotypes. Food Chem. 100, 1217–1219 (2007).
Shojaaddini, M., Moharramipour, S. & Sahaf, B. Fumigant toxicity of essential oil from Carum copticum against Indian meal moth, Plodia interpunctella. J. Plant Protect. Res. https://doi.org/10.2478/v10045-008-0050-5 (2008).
Dhaiwal, K., Chahal, K. K., Kataria, D. & Kumar, A. Gas chromatography-mass spectrometry analysis and in vitro antioxidant potential of ajwain seed (Trachyspermum ammi L.) essential oil and its extracts. J. Food Biochem. 41, e12364 (2017).
Lotfaliani, M., Ayatollahi, S. A., Kobarfard, F. & Pour, P. M. Chemistry, biological activities and toxic effects of alkaloidal constituents of genus Delphinium—A mini review. J. Herbmed. Pharmacol. 10, 486–499 (2021).
Kelly, P. H., Yingling, A. V., Ahmed, A., Hurwitz, I. & Ramalho-Ortigao, M. Defining the mechanisms of action and mosquito larva midgut response to a yeast-encapsulated orange oil larvicide. Parasites Vect. 15, 183 (2022).
Cruz-Castillo, A. U. et al. Terpenic constituents of essential oils with larvicidal activity against Aedes Aegypti: A QSAR and docking molecular study. Molecules 28, 2454 (2023).
Isman, M. B. & Tak, J. H. Inhibition of acetylcholinesterase by essential oils and monoterpenoids: a relevant mode of action for insecticidal essential oils? Biopest. Int. 13, 71–78 (2017).
Pavela, R., Maggi, F., Iannarelli, R. & Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Tropica 193, 236–271 (2019).
Pavela, R. & Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 21, 1000–1007 (2016).
Nawaz, M., Mabubu, J. I. & Hua, H. Current status and advancement of biopesticides: Microbial and botanical pesticides. J. Entomol. Zool. Stud. 4, 241–246 (2016).
Mathew, L. K. Botanicals as biopesticides: A review. Int. J. Adv. Res. 4, 1734–1739 (2016).
Acknowledgements
Our appreciation goes to Dr. A.A. Keshtkar, Dr. A. Latifi, Dr. A. Ebrahimi, and Mr. A. Azadnia for his direction and counsel throughout the project.
Funding
This work was founded and supported by the Maragheh University of Medical Sciences (MRGUMS), (IR.MARAGHEHPHC.REC.1401.012) Maragheh, Iran.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.R., H.N., and A.S-D.; Methodology, H.N., M.P., and A.S-D.; Formal Analysis, S.R., and M-B.B.; Investigation, S.R., A-M.T., and H.N.; Data Curation, S.R., E.N., and M-B.B.; Writing—Original Draft Preparation, S.R., A.S-D., M-B.B., and A-M.T.; Writing—Review & Editing, S.R., H.N., A.S-D., M-B.B., A-M.T., E.N., and M.P.; Supervision, H.N.; Project Administration, S.R.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sanei‑Dehkordi, A., Tagizadeh, A.M., Bahadori, M.B. et al. Larvicidal potential of Trachyspermum ammi essential oil and Delphinium speciosum extract against malaria, dengue, and filariasis mosquito vectors. Sci Rep 14, 20677 (2024). https://doi.org/10.1038/s41598-024-71829-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71829-x