Abstract
Fundamental changes in intracellular processes, such as overactive growth signaling pathways, are common in carcinomas and are _targets of many cancer therapeutics. GRIP and coiled-coil containing 2 (GCC2) is a trans-Golgi network (TGN) golgin maintaining Golgi apparatus structure and regulating vesicle transport. Here, we found an aberrant overexpression of GCC2 in non-small cell lung cancer (NSCLC) and conducted shRNA-mediated gene knockdown to investigate the role of GCC2 in NSCLC progression. shRNA-mediated GCC2 knockdown suppressed NSCLC cell growth, migration, stemness, and epithelial-mesenchymal transition (EMT) in vitro and tumor growth in vivo. In addition, GCC2 knockdown suppressed cancer cell exosome secretion and the oncogenic capacity of cancer cell-derived exosomes. Mechanistically, GCC2 inhibition decreased epidermal growth factor receptor (EGFR) expression and downstream growth and proliferation signaling. Furthermore, GCC2 inhibition compromised Golgi structural integrity in cancer cells, indicating a functional role of GCC2 in regulating intracellular trafficking and signaling to promote lung cancer progression. Together, these findings suggest GCC2 as a potential therapeutic _target for the treatment of NSCLC.
Similar content being viewed by others
Introduction
Lung cancer, the leading cause of cancer-related deaths in the United States, is estimated to be responsible for 127,070 deaths in 2023, which is far more than any other cause of cancer mortality 1. More than 80% of lung cancer cases are classified as non-small cell lung cancer (NSCLC), and the 5-year relative survival rate for NSCLC is 23% 2. While recent advances in diagnosis and treatment options have improved the outlook on lung cancer, prognosis remains unfavorable for lung cancer patients. Therefore, understanding the molecular mechanisms of lung cancer progression and developing novel therapeutic _targets are crucial to improve patient survival.
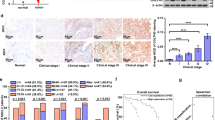
GRIP and coiled-coil containing 2 (GCC2) is a trans-Golgi network (TGN) golgin with a conserved GRIP domain and an extensive coiled-coil domain to form rod-like, α-helical homodimers. GCC2 protein is involved in vesicle trafficking and the maintenance of Golgi apparatus structure 3. For instance, GCC2 has been shown to mediate Rab9-dependent vesicle tethering and recycling of mannose 6-phosphate receptors from late endosomes to Golgi 4. In addition, GCC2 has a functional domain required for maintenance of Golgi structure 5. The Golgi apparatus is a dynamic organelle involved in anterograde and retrograde vesicle trafficking, cargo sorting and secretion, and cellular processes, including mitosis, cell polarity and motility, autophagy, apoptosis, and inflammation 6,7,8. Alterations in Golgi apparatus structure and Golgi-mediated processes, which involves golgins, have been linked to cancer growth and metastases 9,10,11,12. For example, our previous study revealed increased exosome secretion and elevated GCC2 expression in both NSCLC patient plasmas derived from various pathological stages and NSCLC cell lines, suggesting GCC2 as a novel, non-invasive biomarker for early detection of NSCLC 13. Tu et al. reported that GCC2 knockout had anti-tumor effects by interrupting post-Golgi trafficking of STING, activating tonic interferon signaling, and ultimately promoting anti-tumor immune responses, and another study demonstrated GCC2-ALK as a _targetable fusion in NSCLC 14,15,16. While other Golgi-associated proteins, such as Golgi phosphoprotein 3 (GOLPH3), Golgi membrane protein 1 (GOLM1), GM130, and golgin-97, have been linked to tumorigenesis, further insight is needed on whether GCC2 expression affects NSCLC progression, and a better understanding of the underlying mechanisms could provide a basis for the development of novel cancer therapies 17,18,19,20,21.
Epidermal growth factor receptor (EGFR), a member of the receptor tyrosine kinase (RTK) superfamily, is a transmembrane protein with an extracellular domain binding growth factors and an intracellular kinase domain initiating the mitogen-activated protein kinase (MAPK) cascade 22. Mutated EGFR is a common oncogenic driver in cancers, including NSCLC, and it is responsible for uncontrolled proliferation and malignant transformation of cancer cells 23. ERK, a component of the canonical Raf-MEK-ERK MAPK signaling cascade, can activate cytoplasmic and nuclear substrates to stimulate cell proliferation, movement, survival, and immune responses 24,25. For example, phosphorylated ERK1/2 translocates to the nucleus and acts on transcription factors Elk, c-Fos and Myc for the expression of cyclin D1, a regulator of cell cycle progression. Increased EGFR expression and signaling contributes to tumor growth, metastasis, resistance to chemotherapy, and overall worse prognosis; consequently, EGFR tyrosine kinase inhibitors (EGFR-TKI) have been developed and are used as a first-line treatment for patients with NSCLC 26. The inhibition of EGFR and downstream signaling pathways remain a favorable _target for the treatment of lung cancer. Since the TGN is an important site for EGFR trafficking and signaling, there is a potential role of GCC2 in regulating EGFR signaling and NSCLC progression 19,27. Exosomes are nano-sized vesicles with sizes of 30–200 nm and play an important role in intercellular communication 28. Exosome biogenesis begins with the endocytic pathway. Endosome maturation, invaginations forming intraluminal vesicles (ILVs), and multivesicular bodies (MVBs) fusing with the plasma membrane releases ILVs into the extracellular space as exosomes 29. While exosome biogenesis is a complex process that is not fully understood, exosomes have been demonstrated to reflect the characteristics of the origin cell and contain various cargos, such as proteins and non-coding RNAs. Tumor-derived exosomes play critical roles in shaping the tumor microenvironment for tumorigenesis and tumor progression by acting as signaling molecules between cancer cells and surrounding cells 30. Exosomes can initiate epithelial-mesenchymal transition (EMT) in cancer cells and give rise to cancer stem cells (CSC), which are a subset of cells with an unlimited capacity for self-renewal 31.
To the best of our knowledge, there are no studies that have specifically studied GCC2 expression and NSCLC progression 13,14. In this study, we investigated how GCC2 overexpression affects NSCLC progression and elucidated potential molecular mechanisms to suggest GCC2 as a promising therapeutic _target for the treatment of NSCLC.
Results
GCC2 regulates cell proliferation, invasion, and migration in NSCLC cell lines
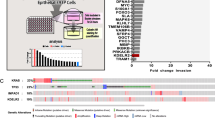
First, we analyzed GCC2 protein expression levels in various NSCLC cell lines and a pulmonary alveolar epithelial cell line, and we found H460 and H1299 cell lines had the highest GCC2 expression levels (Supplementary Fig. S1A). To investigate the role of GCC2 in lung cancer cell lines, we inhibited the expression of GCC2 in two NSCLC cell lines with the highest levels of GCC2 protein, H460 and H1299. We confirmed the effects of shRNA-mediated GCC2 knockdown, as GCC2 mRNA and protein expression in both NSCLC cell lines decreased by more than 70% relative to control (Fig. 1A and B, Supplementary Fig. S1B and C). There were noticeable differences in cell counts following GCC2 knockdown, so we analyzed the cell viability of H460 cells after shGCC2 treatment in a time-dependent manner (Fig. 1C). GCC2 knockdown H460 cells had a 50% reduction in cell viability 8 h post-transfection, and this effect was maintained for another 16 h. H460 cells with decreased GCC2 expression demonstrated a tenfold increase of TUNEL-positive cells and similar results were seen in H1299 cells, suggesting apoptosis induction in both NSCLC cell lines following GCC2 knockdown (Fig. 1D, Supplementary Fig. S1D). These results suggest shRNA-mediated GCC2 knockdown impairs proliferation and survival of H460 and H1299 cells.
GCC2 knockdown inhibits NSCLC proliferation, migration, invasion, and survival. (A) RT-qPCR analysis and (B) western blot analysis of relative GCC2 expression levels in H460 cells following transfection with shGCC2. (C) Cell viabilities of GCC2 knockdown H460 cells were measured by MTS assay at 0, 4, 8, 12, and 24 h. (D) Detection of TUNEL-positive cells in H460 cells transfected with shGCC2 plasmids. Scale bar = 50 μm. RT-qPCR analysis of relative gene expression of (E) CSC-related biomarkers and (F) EMT-related genes. (G) Colony formation assays of control and GCC2 knockdown H460 cells with colony counts summarized on the right. (H) Wound-healing assay measured at 0 and 48 h demonstrating the migratory potential of control and GCC2 knockdown H460 cells. Scale bar = 100 μm. (I) Transwell migration and invasion assays in control and GCC2 knockdown H460 cells. Scale bar = 100 μm. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Next, we explored the changes in gene expression patterns of NSCLC cell lines following GCC2 knockdown. Cancer stem cells (CSCs) are a subpopulation of cancer cells with self-renewal and differentiation capacities similar to those of stem cells, and they play a significant role in tumor initiation, relapse and resistance to treatments 32. qPCR analysis demonstrated GCC2 knockdown cells had expression of CSC-related genes, such as OCT4 and SOX2, decrease by more than 50% relative to control cells (Fig. 1E, Supplementary Fig. S1E). The process of adherent epithelial cells acquiring mesenchymal characteristics and migratory properties is known as epithelial-mesenchymal transition (EMT). EMT is a critical process linked with stem-cell characteristics and metastatic progression in cancers and has specific, identifiable changes in gene expression 33. Silencing of GCC2 expression in NSCLC cell lines decreased mRNA expression of mesenchymal-associated genes, such as Snail, Slug and Zeb, while expression of E-cadherin, a marker of epithelial state, in H460 cells was unchanged (Fig. 1F, Supplementary Fig. S1F). Having observed changes in CSC- and EMT-related genes at the mRNA level, we investigated whether there were concomitant changes in cancer cell phenotypes. shGCC2-treated NSCLC cell lines showed decreased colony formation abilities (Fig. 1G, Supplementary Fig. S1G). GCC2 knockdown inhibited the migratory potential of cancer cells, demonstrated by a lowered capacity for gap recovery in the scratch assay in H460 and H1299 cells (Fig. 1H, Supplementary Fig. S1H). In addition, GCC2 knockdown H460 and H1299 cells exhibited approximately 85% and 60% decreases, respectively, in cell movement for transwell migration and invasion assays (Fig. 1I, Supplementary Fig. S1I). Therefore, shRNA-mediated silencing of GCC2 in lung cancer cell lines decreased CSC- and EMT-related gene expression profiles, which were further supported by inhibition of cancer cell growth and metastatic potential in vitro.
GCC2 regulates the secretion and pro-tumor effects of cancer cell-derived exosomes
Formation and cargo sorting of early sorting endosomes, which become MVBs that secrete exosomes, are regulated by the trans-Golgi network 34. Therefore, we investigated the effects of inhibiting GCC2 expression on exosome secretion. Nanoparticle tracking analysis (NTA) results demonstrated a decrease in number of total exosomes released from GCC2 knockdown H460 cells relative to control (Fig. 2A). Additionally, immunostaining for CD63, a tetraspanin protein and an exosome marker, revealed a significant decrease in CD63 + green fluorescence puncta upon GCC2 knockdown in H460 and H1299 cell lines (Fig. 2B). Here, GCC2 gene silencing inhibited exosome secretion in H460 cells.
GCC2 knockdown decreases exosome release and abolishes the migration- and proliferation-promoting effects of cancer cell-derived exosomes. (A) Total number of exosomes released in control and GCC2 knockdown H460 cells were determined by nanoparticle tracking analysis. (B) Immunofluorescence results of CD63-positive exosomes (green) in H460 and H1299 cells. Cell nuclei were stained with Hoechst. Scale bar = 10 μm. H460 cell-derived, GCC2 + exosomes were collected and measured by nanoparticle tracking analysis, and H460 cells were treated with GCC2 + exosomes in a dose-dependent manner for 48 h. (C) Heat-map indicating the changes in mRNA expression levels of CSC- and EMT-related genes of H460 cells treated with GCC2 + exosomes. (D) Transwell migration and invasion assays were performed to assess the migratory ability of H460 cells treated with GCC2 + exosomes. Scale bar = 100 μm. (E) Simultaneously, colony formation assays were performed in H460 cells treated with GCC2 + exosomes. GCC2 knockdown H460 cell-derived, GCC2 KD exosomes were isolated and treated on H460 cells in a dose-dependent manner. (F) Heat-map indicating changes in mRNA expression levels of CSC- and EMT-related genes in H460 cells treated with GCC2 KD exosomes. (G) Transwell migration and invasion assays were performed to assess the migratory ability of H460 cells following GCC2 KD exosome treatment. Scale bar = 100 μm. (H) Colony formation abilities of H460 cells treated with GCC2 KD exosomes. (I) Graphs comparing results of migration, invasion, and colony formation assays of H460 cells treated with either GCC2 + or GCC2 KD exosomes in increasing doses. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Cancer cell-derived exosomes not only confer pro-tumorigenic traits to surrounding cancer cells, but also are diagnostic, predictive, and prognostic biomarkers 30,31. Jeong et al. identified GCC2-containing exosomes as a biomarker for NSCLC progression, and exosomal GCC2 protein levels increased with pathological stage, suggesting GCC2 enrichment in NSCLC-derived exosomes 13. Therefore, we investigated the role of NSCLC cell-derived, GCC2 enriched exosomes, which will hereafter be denoted as “GCC2 + exosomes”, and explored the effects of cancer cell GCC2 expression on exosome functions. Briefly, exosomes were isolated from H460 cells with either normal or silenced GCC2 expression, analyzed for proper isolation, calculated for dosage determination, and treated to cancer cells in an autocrine and dose-dependent manner. Upon treatment of GCC2 + exosomes on H460 cancer cells in increasing doses, we found that there were parallel increases in expression of CSC- and EMT-related genes, such as OCT4, NANOG, CD133, Snail, and Twist (Fig. 2C). Interestingly, once treatment doses exceeded 10x, there were no statistically significant differences in gene expression between 10x, 20x, and 30 × treatment conditions, suggesting a saturation effect of GCC2 + exosomes. At the level of cell phenotype, transwell migration and invasion assays demonstrated a similar dose-dependent, migratory potential promoting effect of GCC2 + exosomes in H460 cells (Fig. 2D). Like the saturation effect observed with gene expression, the migration promoting effects of GCC2 + exosomes appeared maximal at, but not beyond, the 10 × dose. Colony formation abilities demonstrated increasing trends upon treatment with lower concentrations of GCC2 + exosome, and 10 × or more GCC2 + exosomes significantly upregulated colony formation ability in H460 cells (Fig. 2E). Together, these results demonstrated GCC2 + exosomes promote cancer cell proliferation and migration.
To investigate the relationship between GCC2 expression and functions of cancer cell-derived exosomes, we first inhibited GCC2 expression in cancer cell lines, isolated GCC2 knockdown cancer cell-derived exosomes, henceforth referred to as “GCC2 KD exosomes”, and treated GCC2 KD exosomes to H460 cells in a dose-dependent manner. Since GCC2 silencing reduced exosome secretion in H460 cells (Fig. 2A and B), we standardized the number of particles treated at each dose to ensure equal treatment conditions for GCC2 + and GCC2 KD exosomes; NTA results were used to set 2.5 × 108 particles as 1x. In addition, dose-dependent treatment of GCC2 KD exosomes on H460 cells was performed until 10x, as evidenced by the saturation effect observed with GCC2 + exosomes. Surprisingly, despite treating the same number of particles as GCC2 + exosomes, GCC2 KD exosomes had a less prominent effect on promoting the expression of CSC- and EMT-related genes in H460 cells (Fig. 2F). Relative to untreated, control conditions, the expression of most genes in H460 cells did not exhibit an increasing response with dose-dependent GCC2 KD exosome treatment. At lower doses, the treatment GCC2 KD exosomes decreased the expression EMT-related genes N-cadherin, Snail, Slug, Zeb, and Twist relative to control in H460 cells. Transwell migration and invasion assays did not reveal a difference in migratory potential of cancer cells treated with GCC2 KD exosomes, no matter the dose (Fig. 2G). Colony formation ability of H460 cells increased only after treatment of 10 × GCC2 KD exosomes, and lower treatment doses did not enhance colony formation abilities (Fig. 2H). The effects of GCC2 + and GCC2 KD exosomes on migration, invasion, and colony formation are summarized in Fig. 2I. Here, we found that silencing GCC2 expression in H460 cells inhibited migration, but not proliferation, promoting effects of GCC2 + exosomes in H460 cells. This suggests GCC2 in cancer cells may regulate cargo sorting of exosomes to contain key factors that alter CSC- and EMT- gene expression and migratory potential in recipient cells.
GCC2 regulates RTK expression and MAPK signaling pathways
Dysregulated growth receptor activity is associated with cancer progression, and binding of growth factors to their cognate receptors have been shown to induce EMT and CSC-like states in cancer cells through MAPK signaling cascades 35,36. Previous results demonstrated GCC2 knockdown decreased CSC- and EMT-related gene expression in lung cancer cell lines, so we investigated whether GCC2 expression was related to the expression of growth receptors and/or downstream signaling pathways. qPCR analysis of various receptor tyrosine kinase (RTK) genes demonstrated decreased expression in H460 and H1299 GCC2 knockdown cells (Fig. 3A, Supplementary Fig. S2A). Immunoblot analysis for MEK1/2 and ERK1/2, both of which are key downstream components of growth receptor signaling cascades, revealed decreased expression and phosphorylation-mediated activation in GCC2 knockdown NSCLC cell lines (Fig. 3B, Supplementary Fig. S2B). In addition to MAPK/ERK signaling factors, the mRNA expression of other MAPK pathway-related genes, such as KRAS and cyclin D1, were decreased upon GCC2 knockdown H460 and H1299 cell lines (Fig. 3C, Supplementary Fig. S2C). Here, GCC2 silencing in NSCLC cell lines decreased expression of multiple RTKs and components of the downstream MAPK/ERK signaling pathway.
GCC2 knockdown decreases various receptor tyrosine kinase expression and downstream MAPK signaling pathway. (A) RT-qPCR analysis of various receptor tyrosine kinases in control and GCC2 knockdown H460 cells. (B) Western blot analysis of MEK, pMEK, ERK, pERK of whole-cell lysates of control and GCC2 knockdown H460 cells. (C) RT-qPCR analysis of MAPK signaling pathway-related genes in control and GCC2 knockdown H460 cells. (D) Western blot analysis of cyclin D1 in whole-cell lysates of control and GCC2 knockdown H460 cells. Immunofluorescence analysis of (E) cyclin D1 (scale bar = 50 μm) and (F) Ki-67 (scale bar = 10 μm) in control and GCC2 knockdown H460 cells with quantification of CCDN1 + and Ki-67 + cells, respectively, relative to Hoechst stained cells. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Cyclin D1, a downstream effector of the EGFR-MAPK signaling cascade, is a key regulator of cell cycle progression, and cyclin D1 is pivotal in the malignant transformation of NSCLC 37. Therefore, we analyzed the expression of cyclin D1 in GCC2 control and knockdown NSCLC cell lines and found decreased cyclin D1 protein levels in GCC2 knockdown cancer cells (Fig. 3D, Supplementary Fig. S2D). Immunostaining for cyclin D1 in H460 cells revealed a more than 50% decrease in CCND1-positive cell counts after GCC2 knockdown, and Ki-67 positive cells decreased by approximately 60% in shGCC2 treated H460 cells (Fig. 3E and F). Similar immunostaining results were seen for both cyclin D1 and Ki-67 in H1299 cells following GCC2 knockdown (Supplementary Fig. S2E and F). Here, we demonstrated GCC2 expression affects RTK expression in cancer cell lines, and GCC2 knockdown reduced MAPK signaling activity, including the downstream effector cyclin D1, suggesting GCC2 may regulate multiple growth signaling pathways.
GCC2 regulates NSCLC tumor growth in vivo
To investigate whether growth-inhibiting effects of GCC2 silencing in vitro could be replicated in vivo, we established a tumor xenograft model in BALB/c nude mice. Immunodeficient mice were inoculated with either control H460 cells expressing GCC2 or shGCC2-treated H460 cells. Mice were observed for 3 weeks after tumor inoculation. Tumor growth was significantly inhibited in mice bearing GCC2 knockdown H460 cells compared to those inoculated with control H460 cells (Fig. 4A). Both tumor volumes and tumor weights had statistically significant decreases in the GCC2 knockdown group at endpoint (Fig. 4B and C). At endpoint, the average tumor size of control H460 cell-derived tumors was 485.7 mm3, whereas the average tumor size of the three remaining GCC2 KD H460 cell-derived tumors was 8.9 mm3, which is more than 50-fold smaller relative to control (Fig. 4A and B). On the other hand, bodyweights of mice remained unchanged between the two groups throughout the experiment (Fig. 4D). In some mice, GCC2 knockdown H460 cells formed tumors soon after inoculation but were no longer detected at endpoint, suggesting impaired survival and growth abilities of GCC2 knockdown cancer cells (Fig. 4B).
GCC2 knockdown inhibits H460 tumorigenesis in vivo. BALB/c nude mice were subcutaneously injected with 1 × 106 GCC2 knockdown or control H460 cells and were sacrificed 21 days after tumor inoculation (n = 5 for each group). (A) Photograph of dissected tumor xenografts from the indicated experimental groups. Tumor analysis for (B) tumor volumes, which were recorded 3 times a week, and (C) tumor weights, which were measured immediately after tumor extraction. (D) Bodyweights of mice were measured over the course of the experiment. (E) Representative images of hematoxylin–eosin stained tumor paraffin sections. Scale bar = 50 μm. (F) Immunohistochemistry analysis for EGFR and cyclin D1 in control and GCC2 knockdown H460 tumor xenografts. Scale bar = 10 μm. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Morphological analysis of tumors with H&E staining not only confirmed the smaller absolute size of GCC2 knockdown cancer cell-derived tumors, but also revealed a less dense population of cells within these tumors (Fig. 4E). Immunohistochemistry analysis revealed decreased expression of EGFR and cyclin D1 in GCC2 knockdown H460 tumor xenografts compared to control counterparts (Fig. 4F). These results support the changes observed in RTK expression and MAPK signaling pathways in Fig. 3. Here, we demonstrated the tumor growth inhibiting effects of GCC2 knockdown in a NSCLC cell line in-vivo.
GCC2 regulates the integrity of Golgi structure and intracellular trafficking of EGFR
GCC2 is involved in the maintenance of Golgi apparatus structure and Golgi-associated processes, and disrupted Golgi structures inhibits the transport and aberrant activity of EGFR 3,38. To investigate the role of GCC2 in Golgi apparatus structure and changes in cellular EGFR activity, we first demonstrated the co-localization of GCC2 and Golgi marker GM130 through immunostaining in H460 and H1299 cell lines (Fig. 5A). There was a clear co-localization of GCC2 and GM130 in a compact structure that is presumed to be the Golgi apparatus. Next, we investigated the changes in Golgi structure after GCC2 silencing in NSCLC cell lines. Immunostaining for GM130 in Fig. 5B demonstrated GCC2 knockdown resulted in a dispersed, less compact Golgi apparatus morphology in both H460 and H1299 cell lines. This suggests, GCC2 expression is associated with maintenance of Golgi structure in NSCLC cell lines.
GCC2 knockdown compromises Golgi apparatus integrity and decreases nuclear translocation of EGFR. Immunofluorescence analysis of (A) GM130 and GCC2 and in H460 and H1299 cells and (B) GM130 in control and GCC2 knockdown H460 and H1299 cells. (C) Western blot analysis of EGFR in control and GCC2 knockdown H460 cells. (D) Immunostaining for EGFR in control or GCC2 knockdown H460 cells; Representative images are shown. Scale bar = 10 μm. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In our previous results, we observed decreased expression of various RTKs in GCC2 knockdown H460 and H1299 cells, so we explored the relationship between GCC2 and EGFR, a common oncogenic driver mutation in cancers that drives CSC and EMT induction. In Fig. 5C and Supplementary Fig. S2G, we confirmed the effects of GCC2 knockdown and decreased EGFR expression at the protein level. qPCR analysis for EGFR in H460 cells treated with GCC2 + or GCC2 KD exosomes revealed EGFR expression increased in a dose-dependent manner upon treatment of GCC2 + exosomes but not GCC2 KD exosomes (Supplementary Fig. S2H). Nuclear translocation of EGFR allows EGFR to act as a co-transcription factor to promote cell proliferation and cancer progression 35. Control H460 cells expressing GCC2 have significant amounts of EGFR residing in the nucleus region, as demonstrated by red puncta in immunostaining results (Fig. 5D). On the contrary, GCC2 knockdown H460 cells had a drastic decrease of EGFR localized in the nucleus, suggesting aberrant GCC2 overexpression in cancer cells may promote the transport of EGFR into the nucleus for cancer progression.
Discussion
NSCLC is a highly heterogeneous malignancy and remains a leading cause of cancer-related mortalities. Despite recent advances in early diagnosis and treatment methods, resistance to therapy and tumor relapse challenges the treatment of NSCLC. Therefore, exploring novel therapeutic _targets and understanding cancer-associated cellular processes could provide an opportunity for improved patient prognosis. In this study, we investigated the role of GCC2 as an oncogene in NSCLC and demonstrated two major findings. First, aberrant overexpression of GCC2 gene is involved in cancer cell exosome secretion, as well as the tumor-promoting effects of exosomes. Dose-dependent treatment of GCC2 + exosomes enhanced colony formation, invasiveness, and migratory potential, while such effects were ablated upon treatment of GCC2 KD exosomes. Second, inhibition of aberrant GCC2 overexpression in NSCLC cell lines decreased the proliferative and migratory capacity of H460 and H1299 cells, and underlying mechanisms involves the regulation of intracellular EGFR trafficking and MAPK signaling cascades. Together, our results suggest GCC2 is a regulator of key tumorigenic processes in NSCLC.
In this study, we demonstrated GCC2 knockdown inhibits cell proliferation and promotes apoptosis in cancer cells, and decreased GCC2 expression suppresses CSC- and EMT-related gene expression and related cell phenotypes. Mechanistically, we observed downregulated expression of multiple RTKs upon GCC2 knockdown and further confirmed decreased signaling through the canonical MAPK/ERK signaling cascade. ERK1/2 is a central component of MAPK signaling that regulates numerous biological processes within the cell. For example, ERK1/2 controls G1 to S phase transition by inducing the expression of cyclin D1, inhibits apoptosis by suppressing proapoptotic factors and stimulating antiapoptotic proteins, regulates the renewal capacity of cancer cells, and reorganizes cytoskeletal elements for productive cell migration 39. Previous studies have demonstrated that phosphorylation of ERK is critical for nuclear translocation and downstream signaling outcomes, as ERK phosphorylation releases the kinase from cytosolic anchors and reveals key Ser residues that can associate with importin7 for transport through the nuclear envelope 40,41,42. Thus, phosphorylation induces conformational changes of residues in the activation loop of ERK to control subcellular localization, and non-phosphorylatable ERK mutants are not able to translocate in the nucleus 41. Our results showed GCC2 knockdown in H460 and H1299 cells decreased pERK levels, suggesting impaired nuclear translocation of ERK that promotes gene expression and cell proliferation. GCC2 knockdown in NSCLC cells downregulated cyclin D1 expression at the mRNA and protein levels and decreased cyclin D1 immunofluorescence signals, indicating impaired ERK1/2 signaling decreases cyclin D1 activity. Cyclin D1 is a key regulator of G1 to S phase transition, as depletion of cyclin D1 results in G1 arrest 43. These results support the phenotypic changes observed in colony formation assays. Furthermore, decreased proportion of Ki-67 positive cells following GCC2 knockdown supports impaired cell cycle progression. Cyclin D-CDK4/6 complexes promote entry into the S phase through the retinoblastoma protein and E2F-mediated transcription of Ki-67 44. Ki-67 undergoes proteasomal degradation in G0 and G1 phases and accumulates during S, G2, and M phases, so Ki-67 is maximally expressed in G2 phase and mitosis 45. Therefore, decreased cyclin D1 expression and Ki-67 positive cells after GCC2 knockdown suggests cancer cells are unable to progress beyond the G1/S transition of the cell cycle. Our findings suggest GCC2 promotes cancer cell proliferation, migration, and survival by facilitating RTK activity, downstream ERK1/2 activation, and cell cycle progression. Moreover, experiments in BALB/c nude mice confirmed the tumor growth-promoting activity of GCC2 in-vivo.
Aberrant alterations of the Golgi apparatus, a signaling hub involved in the processing and trafficking of proteins for secretory and endocytic pathways, have been linked to cancer progression 9,11. Golgi proteins, such as GOLPH3 and GM130, have been demonstrated to promote tumorigenesis as potential oncogenes in various cancers 18,46,47. Studies have shown increased expression of GOLM1 promotes NSCLC cancer progression by regulating P53 in MAPK signaling and upregulating EMT, and Ye et al. demonstrated GOLM1 as a regulator of endocytic EGFR trafficking to promote receptor recycling and prolong growth factor signaling to induce HCC metastasis 19,48,49. In a similar manner, we speculate GCC2, which is upregulated in NSCLC and drives cancer progression, is involved in EGFR trafficking and nuclear translocation. Activated EGFR is internalized and subject to different fates, such as recycling back to the plasma membrane in Rab11 + endosomes, trafficking to lysosomes for degradation, or retrograde translocation through the Golgi apparatus and ER to the nucleus 27,50. Nuclear translocation of EGFR is mediated by Sec61, and EGFR residing in the nucleus has trans-activating activity for the expression of E2F1, CCND1, and Ki-67 51. In a similar regard, ERK1/2 activates cytosolic substrate ribosomal S6 kinase (RSK) and nuclear substrate Elk1 to regulate the expression of growth and proliferation genes 52. In this study, the nuclear translocation of EGFR was compromised in GCC2 knockdown H460 cell lines and tumor xenografts, as illustrated by decreased red puncta in the nuclear region of cells in Fig. 4F and 5D. Therefore, the downregulation of aberrant GCC2 overexpression in NSCLC cell lines inhibited the expression and activity of EGFR and ERK1/2, implicating GCC2 in cancer cell growth signaling. We suggest a model where GCC2 knockdown induces Golgi fragmentation, compromises Golgi structural integrity, and dysregulates Golgi functions to impair EGFR nuclear translocation and downregulate pro-tumorigenic signaling in lung cancer cells.
The other major finding of this study is GCC2 knockdown reduced biogenesis and secretion of exosomes in NSCLC cells, as demonstrated through NTA results and CD63 immunostaining. Accumulating evidence suggests exosomes promote malignant tumor progression, whether that’s by controlling immune responses, regulating angiogenesis, inducing proliferation and metastasis, or promoting drug resistance 31,34. In this study, we demonstrated dose-dependent treatment of GCC2 + exosomes promoted CSC and EMT-related gene expression, which were paralleled by changes in colony formation and invasion/migration assays. Furthermore, we found that dose-dependent treatment of GCC2 + exosome increased EGFR expression in recipient H460 cells, suggesting GCC2 + exosomes may promote recipient cell growth signaling pathways. These effects were ablated upon the treatment of GCC2 KD exosomes derived from shGCC2 treated cancer cells. However, at the 10 × dose of GCC2 KD exosomes, we noticed a statistically significant increase in colony formation abilities. We speculate other exosomal cargo, such as miRNAs and proteins, accumulate significantly at the 10 × dose and increase colony formation and proliferation 53. The saturation effects at the 10 × dose of GCC2 KD exosomes may have useful experimental and clinical implications. For example, the 10 × dose may represent an upper threshold of signaling turnover and exosome intercellular communication, and this limit may be useful for determining clinical treatment doses in exosome-based therapeutics.
While there potentially may be other effects of GCC2 + exosomes on cancer progression, such as altering recipient cell apoptosis status or modulating intracellular signaling, the key finding of this study is that GCC2 expression in NSCLC cells regulates the release and composition of exosomes. Exosomal protein composition analysis of GCC2 + and GCC2 KD exosomes revealed GCC2 KD exosomes had decreased GCC2 and EGFR protein levels (Supplementary Fig. S2I). GCC2 gene knockdown in origin H460 cells not only hindered overall exosome secretion, but also decreased GCC2 and EGFR protein content in released exosomes, suggesting GCC2 participates in exosome cargo sorting. Kim et al. reported syntenin-1, a multifunctional adapter protein, promoted the release of small extracellular vesicles (sEV) and the loading of onco-miRNA cargo that enhanced lung cancer proliferation, migration, and angiogenesis 54. Additionally, the Rab family of small GTPases are involved important steps of intracellular vesicle trafficking, exosome biogenesis, and cargo sorting 55. For example, Rab27 regulates MVB docking at the plasma membrane and exocytosis 56. The coiled-coil domains of GCC2 have predicted binding sites for various Rab proteins, including Rab27, so GCC2 potentially interacts with Rab GTPases to regulate exosome secretion, cargo sorting, and recipient tumor cell behavior57. Further investigations on the role of GCC2 expression in exosomes secretion and tumorigenic effects on recipient cells could reveal new insights about the mechanisms of cancer progression.
This study has a few limitations. First, most in vitro experiments were conducted in two NSCLC cell lines, but in vivo tumor xenograft formation was only carried out with H460 cells. Similar anti-proliferative effects in vivo needs to be verified using other NSCLC cell lines. Moreover, a larger and more rigorous animal study would help validate the results of this study. Second, while GCC2 + and GCC2 KD exosome treatment on H460 cells demonstrated differences in pro-tumorigenic effects, we have not elucidated the responsible exosomal components. Proteomic analysis of exosome by LC/MS could reveal new insights about exosomal factors promoting NSCLC progression, as well as the underlying molecular and signaling mechanisms. This approach is similar to other previous studies that have identified the role and function of exosomal proteins. Nonetheless, the current study demonstrated the role of GCC2 expression in lung cancer progression by regulating both EGFR and exosome-mediated signaling. The next step would be to identify responsible factors in exosomes. As such, it would be important to verify the effects by delivering specific factors that can reproduce cancer phenotypes 58,59. In the end, we aim to develop novel therapies based on the findings of the current and future studies, _targeting GCC2 for the management and treatment of NSCLC.
In summary, GCC2 plays an important role in the EGFR/ERK/CCND1 signaling axis and regulates the release and oncogenic potential of cancer cell-derived exosomes. shRNA-mediated GCC2 knockdown decreased proliferation and migration in NSCLC cell lines. Loss of GCC2-mediated vesicle trafficking, impaired EGFR intracellular localization, and downregulation of ERK1/2-mediated cancer signaling are possibly responsible for these effects. GCC2 silencing decreased exosome release and the proliferation- and migration-promoting effects of NSCLC cell-derived exosomes, suggesting GCC2 has multifunctional roles in tumor cell exosome biogenesis and intercellular communication. Collectively, we suggest GCC2 promotes NSCLC progression, and GCC2 may be a potential therapeutic _target for inhibiting the growth of human lung cancer.
Materials and methods
Cell culture
Human pulmonary alveolar epithelial cells (HPAEpiC) were purchased from ScienCell Research Laboratories and cultured in AEpiCM (ScienCell Research Laboratories, CA, USA) with EpiCGS growth supplement, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. NSCLC cell lines, H1299 and H460, were purchased from Korean Cell Line Bank, and were cultured in RPMI-1640 with 5% FBS and 1% P/S. All cells were cultured in a humidified 5% CO2 atmosphere at 37 °C.
GCC2 knockdown by shRNA vector transfection
To perform GCC2 knockdown, the TRC lentiviral GCC2 shRNA vector was purchased from Dharmacon (Lafayette, CO, USA). NSCLC cell lines were seeded to achieve 50–80% confluency one day prior and transfected the following day with the shGCC2 plasmids using Lipofectamine 2000 based on the manufacturer’s protocol.
Quantitative real-time PCR (qRT-PCR)
Total RNAs from H460 and H1299 cells were extracted with TRIzol reagent according to manufacturer’s instructions. cDNA was synthesized using 5 μg of total RNA by reverse transcriptase and oligo dT primers. qRT-PCR was performed in triplicate with KAPA SYBR FAST ABI Prism qPCR kit reagents (KAPA Biosystems, Burlington, MA, USA) using the StepOnePlus Real-Time PCR system. The primers for the genes of interest and cDNA synthesis were synthesized by Cosmogenetech (Seoul, Republic of Korea). Details of all primers used are listed in Supplementary Table 1.
Scratch assay
H460 and H1299 cells were transfected with shCC2 plasmids and cultured in a 6-well plate for 24 h to achieve 90% confluence. A scratch was made across the well using a 200 μl sterile pipette tip. Cells were then incubated in a humidified 5% CO2 incubator at 37 °C with fresh media and observed at 0, 24, and 48 h time points. The scratch gap widths were measured at three different positions and compared with the gap width at 0 h. Distance was measured manually using ImageJ software.
Colony formation assay
H460 and H1299 cells transfected with control or shGCC2 plasmids were seeded into six-well plates at a density of 1,000 cells/well. Cells were incubated for 10–14 days, fixed with methanol, and stained with crystal violet for 10 min. Colony counts were manually measured using ImageJ software.
Transwell migration and invasion assay
Cell migration and invasion assays were performed using Transwell chambers with 8 um pore polycarbonate membrane inserts (Corning, NY, USA). The transwells were coated with collagen (10 μg/ml in H2O) for migration assays and Matrigel for invasion assays. H460 and H1299 cells were seeded in upper chambers with 3,000 cells/well in serum-free medium, and the bottom wells were filled with 200 ul of medium containing 10% FBS. After 24 h incubation at 37 °C, cells were fixed with methanol and stained with crystal violet. Cells were quantified using ImageJ software.
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining
TUNEL assay was performed using a in situ cell death detection kit according to the manufacturer’s instructions (Roche, Mannheim, Germany). Briefly, control and GCC2 knockdown H460 and H1299 cells were fixed for 1 h in 4% paraformaldehyde at room temperature. Following specific labelling, nuclear staining was performed with Hoechst 33342 (Thermo Scientific, Waltham, MA, USA) in phosphate buffered saline (PBS) for 5 min at room temperature. TUNEL-positive cells were defined as those present with fluorescein-dUTP staining.
Cell viability assay (MTS)
H460 cells were seeded in 96-well plates at 4,000 cells/well and transfected with control or shGCC2 plasmids on the following day. MTS reagents (CellTiter96 Aqueous MTS reagent powder, Promega, WI, USA) were added at each 4, 8, 12, 24 h post-transfection and absorbances were triplicate-measured by microplate reader at 490 nm. Data are presented as percentage of viability relative to control cells.
Western blot
NSCLC cell lines and HPAEpiCs were washed with ice-cold phosphate buffered saline, lysed with RIPA lysis buffer containing proteinase inhibitor cocktail mix and total protein extract was quantified using the Bradford assay. Prepared protein samples were separated through SDS-PAGE and transferred onto a nitrocellulose membrane (Amersham Protran 0.45 um NC, Amersham, UK). Membranes were blocked in 5% bovine serum albumin (BSA) and incubated with primary antibodies at 4 °C, overnight. After washing in PBS, the membranes were incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature, washed, and exposed to Clarity Western ECL substrate. Bands were quantified using ImageJ Software. β-actin (Santa Cruz, TX, USA) were used as a loading control. Details of the antibodies used are listed in Supplementary Table S2.
Immunofluorescence analysis
H460 and H1299 cells were seeded on glass cover slips in 24-well plates. Cells were transfected with mock or shGCC2 plasmids. 24 h post-transfection, cells were fixed in 4% paraformaldehyde for 20 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS for 30 min. Following permeabilization, cells were blocked with 5% BSA for 1 h at room temperature and incubated with primary antibodies overnight at 4 °C. After three washes with PBS, cells were incubated with appropriate conjugated secondary antibodies for 1 h at room temperature in the dark. Cells were washed three times with PBS, stained with Hoechst 33,342 for 5 min and mounted using Vectashield (Vector Labs, Burlingame, CA, USA). Immunofluorescence was observed on a Leica DM6 B upright fluorescence microscope (Leica Microsystems, Wetzlar, Germany) or FV1000 confocal microscope (Olympus, Shinjuku, Japan). Details of the antibodies used are listed in Supplementary Table S2.
Exosome isolation and nanoparticle tracking analysis (NTA)
1 × 106 H460 cells were seeded and incubated in exosome-depleted, complete RPMI media for 48 h before collection of conditioned medium. Exosomes were isolated from conditioned medium as described in our previous works 13. Briefly, conditioned medium was centrifuged at 1000 × g for 5 min at 4 °C to remove cell debris, supernatant was passed through 0.2um membrane filter, and transferred to Amicon Ultra-15 centrifugal filters with a molecular weight cut-off of 100 kDa. Filtered supernatant was centrifuged at 5,000 × g for 15 min at 4 °C, and resulting supernatant was collected for another round of centrifugation at 10,000 × g for 30 min at 4 °C. Finally, exosomes were isolated using EXo-I columns (EXoPERT, Seoul, Republic of Korea) according to manufacturer’s instructions and characterized by nanoparticle tracking analysis (NTA) using NanoSight NS300 (Malvern Panalytical, Malvern, UK).
In vitro exosome treatment
Exosomes isolated from either H460 control or shGCC2-treated cells were treated to H460 cells in a dose-dependent manner. Here, we considered the total number of exosomes isolated from 30 mL of conditioned medium as 1x, which contained 2.5 × 108 particles. H460 cells were cultured in exosome-depleted, complete media with or without the addition of exosomes for 48 h.
Tumor xenograft mouse model
Five-week-old pathogen-free conditioned male BALB/c nude mice were obtained from Orient Bio (Seongnam, Republic of Korea). Mice were housed in groups of 5 per cage in a 12 h light/dark cycle with controlled room temperature and relative humidity. NSCLC tumor xenografts were established using control or shGCC2-treated H460 cells. For both control and treatment groups, H460 cell suspensions (1 × 106 cells in 500ul media with equal volume Matrigel) were injected subcutaneously into the dorsal flank of BALB/c syngeneic mice (n = 5 for each group). Tumor size was measured three times weekly using a vernier caliper. Tumor volume (V) was calculated with: V = 1/2 × Length x Width2. Mice were euthanized 21 days after tumor injection using 100% CO2 at a chamber fill rate of 30–70% per minute. After confirmation of death, tumor tissues were isolated and analyzed for further experiments. All animal experiments were performed according to guidelines and regulations and were approved by the Korea University Institutional Animal Care & Use Committee (KUIACUC-2022–0052). We conducted all animal experiments in accordance with ARRIVE guidelines.
Immunohistochemical analysis
Immunohistochemical analysis was performed using formalin-fixed paraffin-embedded tumor xenograft tissues sectioned into 4 µm slices. Slides were deparaffinized using xylene and rehydrated with dilutions of ethanol. Retrieval buffer was used for heat induced epitope retrieval, and sections were permeabilized with 0.25% Triton X-100. Following blocking with 10% normal donkey serum at room temperature for 1 h, the slides were incubated overnight at 4 °C with primary antibodies in 2% normal donkey serum. The slides were washed with PBS and incubated with conjugated secondary antibodies at room temperature for 1 h, and counterstained with Hoechst 33342 for 5 min. Images were captured using a Leica DM6 B upright fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Details of the antibodies used are listed in Supplementary Table S2.
H&E staining
Primary tumor tissues were fixed in 4% formaldehyde overnight, embedded in paraffin, and sectioned into 4 μm slices that were mounted on slide glasses. Tissue sections had paraffin removed by xylene and were gradually rehydrated before hematoxylin and eosin (H&E) staining, which was performed according to manufacturer’s instructions (Abcam, Cambridge, UK).
Statistical analysis
Graphs and statistical analyses were performed using GraphPad Prism 9 software (Dotmatics, CA, USA). Mice were all randomly assigned to treatment groups in animal experiments. All experiments were conducted in triplicates and data are expressed as mean ± SD. Statistical comparisons between groups evaluated using two-tailed Student’s t test or one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Data distributions were assumed to be normal but this was not formally tested. Differences were considered significant when p < 0.05.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Siegel, R. L. et al. Cancer statistics, 2023. Ca Cancer J Clin 73(1), 17–48 (2023).
Schabath, M. B. & Cote, M. L. Cancer progress and priorities: lung cancer. Cancer epidemiology, biomarkers & prevention 28(10), 1563–1579 (2019).
Derby, M. C. et al. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic 8(6), 758–773 (2007).
Reddy, J. V. et al. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Molecular biology of the cell 17(10), 4353–4363 (2006).
Brown, F. C., Schindelhaim, C. H. & Pfeffer, S. R. GCC185 plays independent roles in Golgi structure maintenance and AP-1–mediated vesicle tethering. Journal of Cell Biology 194(5), 779–787 (2011).
Millarte, V. and H. Farhan, The Golgi in cell migration: regulation by signal transduction and its implications for cancer cell metastasis. The Scientific World Journal, 2012. 2012.
Lamb, C. A., Yoshimori, T. & Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nature reviews Molecular cell biology 14(12), 759–774 (2013).
Makhoul, C., Gosavi, P. & Gleeson, P. A. The Golgi architecture and cell sensing. Biochemical Society Transactions 46(5), 1063–1072 (2018).
Bajaj, R. et al. Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells 11(9), 1484 (2022).
Bui, S., et al., Adaptation of the Golgi Apparatus in Cancer Cell Invasion and Metastasis. Frontiers in Cell and Developmental Biology, 2021. 9.
Zhang, X., Alterations of Golgi Structural Proteins and Glycosylation Defects in Cancer. Frontiers in Cell and Developmental Biology, 2021. 9.
Kulkarni-Gosavi, P., Makhoul, C. & Gleeson, P. A. Form and function of the Golgi apparatus: scaffolds, cytoskeleton and signalling. FEBS letters 593(17), 2289–2305 (2019).
Jeong, H. et al. Gcc2 as a new early diagnostic biomarker for non-small cell lung cancer. Cancers 13(21), 5482 (2021).
Tu, X. et al. Interruption of post-Golgi STING trafficking activates tonic interferon signaling. Nature communications 13(1), 6977 (2022).
Qin, J. et al. Rare GCC2-ALK fusion G13: A20 detected by next generation sequencing in non-small cell lung cancer patients and treatment response. Translational Cancer Research 8(5), 2187 (2019).
Jiang, J. et al. GCC2-ALK as a _targetable fusion in lung adenocarcinoma and its enduring clinical responses to ALK inhibitors. Lung Cancer 115, 5–11 (2018).
Scott, K. L. & Chin, L. Signaling From the Golgi: Mechanisms and Models for Golgi Phosphoprotein 3–Mediated Oncogenesis. Clinical Cancer Research 16(8), 2229–2234 (2010).
Scott, K. L. et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 459(7250), 1085–1090 (2009).
Ye, Q.-H. et al. GOLM1 modulates EGFR/RTK cell-surface recycling to drive hepatocellular carcinoma metastasis. Cancer cell 30(3), 444–458 (2016).
Hsu, R.-M. et al. Golgi tethering factor golgin-97 suppresses breast cancer cell invasiveness by modulating NF-κB activity. Cell Communication and Signaling 16, 1–17 (2018).
Baschieri, F. et al. Spatial control of Cdc42 signalling by a GM130–RasGRF complex regulates polarity and tumorigenesis. Nature Communications 5(1), 4839 (2014).
Uribe, M. L., Marrocco, I. & Yarden, Y. EGFR in cancer: Signaling mechanisms, drugs, and acquired resistance. Cancers 13(11), 2748 (2021).
Sharma, S. V. et al. Epidermal growth factor receptor mutations in lung cancer. Nature Reviews Cancer 7(3), 169–181 (2007).
Guo, Y. J. et al. ERK/MAPK signalling pathway and tumorigenesis. Experimental and therapeutic medicine 19(3), 1997–2007 (2020).
Ullah, R., et al. RAF-MEK-ERK pathway in cancer evolution and treatment. in Seminars in cancer biology. 2022. Elsevier.
Nan, X. et al. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Onco_target 8(43), 75712 (2017).
Wang, J., et al., The clathrin adaptor complex-1 and Rab12 regulate post-golgi trafficking of WT epidermal growth factor receptor (EGFR). Journal of Biological Chemistry, 2023. 299(3).
Gurung, S. et al. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Communication and Signaling 19(1), 47 (2021).
Hessvik, N. P. & Llorente, A. Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Sciences 75(2), 193–208 (2018).
Paskeh, M. D. A. et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. Journal of Hematology & Oncology 15(1), 83 (2022).
Dai, J. et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduction and _targeted Therapy 5(1), 145 (2020).
Walcher, L., et al., Cancer Stem Cells—Origins and Biomarkers: Perspectives for _targeted Personalized Therapies. Frontiers in Immunology, 2020. 11.
Ribatti, D., Tamma, R. & Annese, T. Epithelial-mesenchymal transition in cancer: a historical overview. Translational oncology 13(6), 100773 (2020).
Kalluri, R. and V.S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478): p. eaau6977.
Talukdar, S. et al. EGFR: An essential receptor tyrosine kinase-regulator of cancer stem cells. Adv Cancer Res 147, 161–188 (2020).
Lo, H.-W. et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer research 67(19), 9066–9076 (2007).
Gautschi, O. et al. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung cancer 55(1), 1–14 (2007).
Ohashi, Y. et al. _targeting the Golgi apparatus to overcome acquired resistance of non-small cell lung cancer cells to EGFR tyrosine kinase inhibitors. Onco_target 9(2), 1641 (2018).
Chambard, J.-C., et al., ERK implication in cell cycle regulation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2007. 1773(8): p. 1299–1310.
Maik-Rachline, G., Hacohen-Lev-Ran, A. & Seger, R. Nuclear ERK: mechanism of translocation, substrates, and role in cancer. International journal of molecular sciences 20(5), 1194 (2019).
Wolf, I. et al. Involvement of the activation loop of ERK in the detachment from cytosolic anchoring. Journal of Biological Chemistry 276(27), 24490–24497 (2001).
Plotnikov, A., et al., Nuclear extracellular signal-regulated kinase 1 and 2 translocation is mediated by casein kinase 2 and accelerated by autophosphorylation. Molecular and cellular biology, 2011.
Hume, S., Dianov, G. L. & Ramadan, K. A unified model for the G1/S cell cycle transition. Nucleic acids research 48(22), 12483–12501 (2020).
Sobecki, M. et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer research 77(10), 2722–2734 (2017).
Uxa, S. et al. Ki-67 gene expression. Cell Death & Differentiation 28(12), 3357–3370 (2021).
Farber-Katz, S. E. et al. DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell 156(3), 413–427 (2014).
Chang, S.-H. et al. GOLGA2/GM130, cis-Golgi matrix protein, is a novel _target of anticancer gene therapy. Molecular Therapy 20(11), 2052–2063 (2012).
Song, Q. et al. The functional landscape of Golgi membrane protein 1 (GOLM1) phosphoproteome reveal GOLM1 regulating P53 that promotes malignancy. Cell death discovery 7(1), 42 (2021).
Li, L. M. Overexpression of golgi membrane protein 1 promotes non-small-cell carcinoma aggressiveness by regulating the matrix metallopeptidase 13. American journal of cancer research 8(3), 551 (2018).
Sigismund, S., Avanzato, D. & Lanzetti, L. Emerging functions of the EGFR in cancer. Molecular oncology 12(1), 3–20 (2018).
Maisel, S.A. and J. Schroeder, Wrong place at the wrong time: how retrograde trafficking drives cancer metastasis through receptor mislocalization. J. Cancer Metastasis Treat, 2019. 5(7).
Olea-Flores, M. et al. Extracellular-signal regulated kinase: a central molecule driving epithelial–mesenchymal transition in cancer. International journal of molecular sciences 20(12), 2885 (2019).
Sun, Z. et al. Effect of exosomal miRNA on cancer biology and clinical applications. Molecular cancer 17, 1–19 (2018).
Kim, O. et al. Syntenin-1-mediated small extracellular vesicles promotes cell growth, migration, and angiogenesis by increasing onco-miRNAs secretion in lung cancer cells. Cell Death & Disease 13(2), 122 (2022).
Blanc, L. & Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 9(1–2), 95–106 (2018).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology 12(1), 19–30 (2010).
Hayes, G. L. et al. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Molecular biology of the cell 20(1), 209–217 (2009).
Liu, P. et al. Alterations of plasma exosomal proteins and motabolies are associated with the progression of castration-resistant prostate cancer. Journal of translational medicine 21(1), 40 (2023).
Wang, C. et al. Exosomes carrying ALDOA and ALDH3A1 from irradiated lung cancer cells enhance migration and invasion of recipients by accelerating glycolysis. Molecular and cellular biochemistry 469, 77–87 (2020).
Acknowledgements
This work was supported by the Ministry of Science and ICT (2019M3E5D5065399), the National Research Foundation of Korea (NRF-2020R1A2C1101294), and the Ministry of Health and Welfare (RS-2022-00060247) of the government of the Republic of Korea.
Funding
Ministry of Science and ICT, South Korea, 2019M3E5D5065399; National Research Foundation of Korea, NRF-2020R1A2C1101294; Ministry of Health and Welfare, South Korea, RS-2022-00060247.
Author information
Authors and Affiliations
Contributions
Conception and design: M.S.K., H.J., S.H.; acquisition, analysis, and interpretation of data: M.S.K., H.J., J.P., G.S.S., S.H.; manuscript preparation: M.S.K., H.J., S.H.; review and editing: M.S.K., H.J., B.H.C., J.P., G.S.S., J.-H.J., H.S, K.-W.K., O.H.J., J.Y., J.-H.P., Y.P., Y.C., H.K.K., S.H.; funding and final manuscript approval: S.H. All authors gave final approval of the completed version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, M.S., Jeong, H., Choi, B.H. et al. GCC2 promotes non-small cell lung cancer progression by maintaining Golgi apparatus integrity and stimulating EGFR signaling pathways. Sci Rep 14, 28926 (2024). https://doi.org/10.1038/s41598-024-75316-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75316-1