Abstract
Histological assessment is essential for the diagnosis and management of celiac disease. Current scoring systems, including modified Marsh (Marsh–Oberhuber) score, lack inter-pathologist agreement. To address this unmet need, we aimed to develop a fully automated, quantitative approach for histology characterisation of celiac disease. Convolutional neural network models were trained using pathologist annotations of hematoxylin and eosin-stained biopsies of celiac disease mucosa and normal duodenum to identify cells, tissue and artifact regions. Biopsies of duodenal mucosa of varying celiac disease severity, and normal duodenum were collected from a large central laboratory. Celiac disease slides (N = 318) were split into training (n = 230; 72.3%), validation (n = 60; 18.9%) and test (n = 28; 8.8%) datasets. Normal duodenum slides (N = 58) were similarly divided into training (n = 40; 69.0%), validation (n = 12; 20.7%) and test (n = 6; 10.3%) datasets. Human interpretable features were extracted and the strength of their correlation with Marsh scores were calculated using Spearman rank correlations. Our model identified cells, tissue regions and artifacts, including distinguishing intraepithelial lymphocytes and differentiating villous epithelium from crypt epithelium. Proportional area measurements representing villous atrophy negatively correlated with Marsh scores (r = − 0.79), while measurements indicative of crypt hyperplasia positively correlated (r = 0.71). Furthermore, features distinguishing celiac disease from normal duodenum were identified. Our novel model provides an explainable and fully automated approach for histology characterisation of celiac disease that correlates with modified Marsh scores, potentially facilitating diagnosis, prognosis, clinical trials and treatment response monitoring.
Similar content being viewed by others
Introduction
Celiac disease, an autoimmune disease triggered by dietary gluten, affects around 1% of the population1. Its diagnosis can be challenging due to symptom diversity, spanning from no symptoms to severe malabsorption1,2. Patients with celiac disease face a slightly increased overall risk of developing bowel lymphoma in comparison with the general population2. Histological assessment is crucial for the diagnosis and management of celiac disease3, as well as for endpoint assessment in clinical trials4, with findings of intraepithelial lymphocytosis, crypt hyperplasia, and villous atrophy indicative of the presence of the disease5. Clinical evaluations often rely on assessments of disease activity, demonstrated by changes in histology and characterised according to disease severity by classification systems such as the modified Marsh (Marsh–Oberhuber) score and Corazza-Villanacci classification3,6,7, or morphometric approaches that measure villus height, crypt depth, and changes in the villus height to crypt depth ratio8,9. The US Food and Drug Administration recommends using a clinically accepted histological scale such as the Marsh score for screening samples in clinical studies of treatments for celiac disease, to ensure patient eligibility at enrolment. Furthermore, histology is also recommended as a co-primary endpoint in these studies10. However, because inter-observer agreement is low for metrics that rely on qualitative assessments6, the Tampere task force recommends using histological evaluations that employ quantitative measurements, e.g., villous height/crypt depth ratio and percent change in intraepithelial lymphocyte density11.
Celiac disease is often underdiagnosed due to variation between pathologists in their assessment of biopsy tissue samples12, even if multiple biopsies are obtained3,5. Poor quality of biopsy tissue and overlapping histopathology features between related conditions may contribute to this variability5,12,13. Recently, there has been increased interest in applying machine learning (ML) to pathology assessments14,15, including to improve the accuracy and efficiency of celiac disease diagnosis16.
Such automation is expected to significantly reduce variability16,17, enabling smaller clinical studies to attain sufficient statistical power and demonstrate treatment effects. Indeed, convolutional neural network (CNN) tissue and cell model predictions from gastrointestinal samples have been used to create human interpretable features (HIFs) that enable the quantitative assessment of inflammatory pathological changes in non-celiac gastrointestinal diseases18. The application of ML to diagnosis has also been examined for inflammatory bowel disease19.
Although previous research has successfully employed ML to detect celiac disease and assess disease severity based on Marsh scores17, this study aims to bridge critical gaps in the current research landscape. The work presented here represents the first report of an ML application for celiac disease that provides fully explainable tissue segmentation and cell classifications across whole slide images (WSIs) of duodenal mucosal biopsies. Through this approach, we have enabled the extraction of HIFs, such as cell densities, cell count proportions, and tissue area proportions, all of which exhibit correlations with Marsh scores. By utilising ML-based quantification, this study aims to objectively and comprehensively characterise celiac disease histology, address the limitations of manual histological assessments, and provide granular data for translational research and clinical trials. We believe such an approach has tremendous potential as a scalable tool for measuring disease severity and monitoring treatment response.
Materials and methods
Data set characteristics
WSIs of hematoxylin and eosin (H&E)-stained biopsies of duodenal mucosa of varying celiac disease severity (N = 318) and mucosa of normal duodenum (N = 58) were collected from PathAI Diagnostics (Memphis, TN, USA) (Supplemental Fig. 1). Tissues were originally embedded to optimise orientation, and orientation of villi was included as a quality control metric.
The cohort size was determined based on the project’s scope and the availability of small intestine biopsies encompassing the full spectrum of celiac disease histology at the central laboratory (PathAI Diagnostics, Memphis, TN, USA). Slides were scanned at 40 × objective magnification using the Aperio GT450 slide scanner (Leica Biosystems, Wetzlar, Germany). Celiac disease slides were split into training (n = 230; 72.3%), validation (n = 60; 18.9%) and test (n = 28; 8.8%) datasets to ensure the even distribution of available patient metadata. For normal duodenum, slides were divided into a similar ratio of training (n = 40; 69.0%), validation (n = 12; 20.7%) and test (n = 6; 10.3%) datasets.
Patient involvement
The scope of the study concentrated on the analysis of digital pathology images and, as such, direct patient involvement was not a component of the research design, implementation or dissemination plans.
ML-based tissue model development
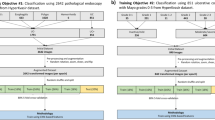
We developed a model to identify and quantify relevant tissue regions, and we also utilised a previously trained model18 to identify and quantify cell types and artifact regions on H&E-stained WSIs of celiac disease and normal duodenum (Fig. 1). Using these identified cells and tissue regions, histological features relevant to celiac disease and representing surrogate measures of modified Marsh score components were quantified, including the proportion of intraepithelial lymphocytes to enterocytes in villous epithelium and the surface areas of villous epithelium and crypt epithelium. The latter two features assess villous height and crypt hyperplasia, respectively.
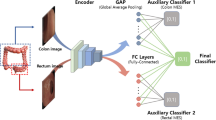
WSIs were annotated by board-certified gastrointestinal pathologists. In total, 8356 tissue region annotations were collected. Annotations of crypt epithelium, villous epithelium, crypt lumen, lamina propria, blood vessels, muscularis mucosa and other tissue (including Brunner’s glands and submucosa) were used to train a HIF-based tissue segmentation model. From these annotations, a CNN model was trained to produce pixel-level predictions of small intestinal mucosa tissue regions. Previously developed models to detect and exclude tissue artifacts and identify and classify the cells in colon tissue were also deployed18. Tissue and cell model predictions were visualised as heatmaps on WSIs. Heatmap transformations were used to remove artifact regions (e.g., debris, tissue folds, out-of-focus regions), extracting features only from high-quality tissue.
Validation and review of cell and tissue models
A PathAI pathologist (FN) performed quality control of the tissue labels used for model training and qualitatively reviewed the tissue and cell overlays representing model predictions on H&E-stained WSIs. This qualitative review helped guide the iterative model development (Supplemental Fig. 2a). Representative overlays for normal duodenum, mild villous blunting, and severe villous blunting are shown in Supplemental Fig. 2b.
Cell and tissue segmentation model overlays showing distinct tissue regions. (a) Overlays generated by cell segmentation model for model deployment. (b) Overlay showing normal duodenum (left) vs. celiac disease (right) in mucosa. (c) Overlay showing normal duodenum (left) vs. celiac disease (right) in all epithelia. (d) Overlay showing normal duodenum (left) vs. celiac disease (right) in villous epithelium. (e) Overlay showing normal duodenum (left) vs. celiac disease (right) in crypt epithelium.
To establish ground truth for cell model prediction accuracy, representative image frames were sampled (75 μm × 75 μm; N = 160). Frames were exhaustively annotated for all model-predicted cell types by five gastrointestinal pathologists. The guidelines for the differentiation of plasma cells, lymphocytes, neutrophils, and eosinophils from H&E-stained images were as follows. Plasma cells were to be identified as cells with an eccentrically placed nucleus with a “clock-face” chromatin pattern, abundant basophilic cytoplasm, and a Golgi apparatus visible as a pale, perinuclear “halo.” Lymphocytes were to be identified as cells with a large, round nucleus with densely clumped chromatin taking up the majority of the cell, scant cytoplasm appearing as a blue rim around the nucleus, and small, uniform overall size. Neutrophils were to be identified as cells with a multi-lobed nucleus connected by thin chromatin strands, a light pink cytoplasm with fine, pale granules, a larger size than a lymphocyte. Eosinophils were to be identified as cells with a bi-lobed or segmented nucleus with consistent spectacled appearance, a cytoplasm filled with large, bright eosinophilic granules. Hierarchical clustering was performed on these annotations and model predictions as previously described to identify cell locations18. To account for potential pathologist bias and variability, Bayesian-estimated ground truths were used to quantify and compare the performance of the annotators and the model (Supplemental Fig. 3).
Evaluation of model-derived HIFs
HIFs (e.g., the proportional area of villous epithelium relative to lamina propria) were extracted from WSIs of normal duodenum (N = 52) and scored celiac disease (N = 118). HIFs were correlated with modified Marsh scores provided by a single gastrointestinal pathologist (type 0, normal lesions; type 1, infiltrative lesions; type 2, hyperplastic lesions; and types 3a, 3b and 3c, destructive lesions)6 using Spearman rank correlations. After establishing correlations between HIFs and modified Marsh scores, potential differences in the model-derived features between celiac disease and normal duodenum were evaluated.
Statistical analysis
To assess cell model performance, sensitivity and specificity were calculated for both the cell model predictions and each pathologist annotation compared with the consensus on representative image frames. To evaluate the model-generated HIFs, each HIF was assessed for correlation with a single pathologist read of the modified Marsh scores using Spearman rank correlations. Data analyses in this study used the programming language Python (OpenEDG Python Institute, West Pomerania, Poland) for tissue and cell model development. Additionally, OpenSlide Python (Carnegie Mellon University, Pittsburgh, PA, USA) was used to load WSIs, Matplotlib (John D Hunter, Matplotlib Development Team and NumFOCUS, Austin, TX, USA) was used for plotting graphs, and PyTorch (PyTorch Foundation, the Linux Foundation, San Francisco, CA, USA) was used for tissue and cell model development. To associate model-derived features of celiac disease following correlations with modified Marsh scores, mean (standard deviation) feature levels were used to show differences between celiac disease and normal duodenum. P values were calculated by independent t-test.
Results
Model development for quantitation of celiac disease histological features
The tissue model developed herein, as well as previously trained cell and artifact models18, were deployed on H&E-stained WSIs of celiac disease and normal duodenum. Relevant cell types identified included neutrophils, plasma cells, enterocytes, intraepithelial lymphocytes, non-intraepithelial lymphocytes, eosinophils and goblet cells (Fig. 2); all other cell types are predicted as “other cells”. In addition, tissue regions identified included villous epithelium, crypt epithelium, lamina propria, muscularis mucosa, and blood vessels. Tissue regions such as total epithelium and mucosa could also be extracted from the tissue segmentation overlays. The tissue model distinguished villous epithelium from crypt epithelium.
The cell model’s performance was evaluated by comparing it with consensus pathologists’ annotations using Bayesian-estimated ground truths. Here, we sought to concentrate this validation on overlapping cells, focusing on cell confusion. The cell model demonstrated acceptable sensitivity for most cell types (Fig. 3a). To determine model accuracy, labels from five gastrointestinal pathologists (Supplemental Fig. 3a) were compared to cell model predictions on representative image frames, as described in Supplemental Fig. 3b. We reported elements of the F1 score for both cell model predictions and pathologists’ labels for each of the cell types (Fig. 3b,c). Overall, cell model specificity remained relatively consistent and was similar to that of the pathologists for most cell types; a slight difference was observed for plasma cells, whereas sensitivity was more variable outside the intraepithelial lymphocyte class.
Correlation of surrogate features with modified Marsh score
HIFs from our models were analyzed to assess correlation with modified Marsh scores, which were provided by a single gastrointestinal pathologist. The area of villous epithelium relative to mucosa was negatively correlated with modified Marsh score (Spearman r = − 0.79, p < 0.0001) (Fig. 4a). The area of crypt epithelium in tissue (Fig. 4b) positively correlated with modified Marsh score (Spearman r = 0.71, p < 0.0001), as did the number of intraepithelial lymphocyte cells relative to enterocyte cells in villous epithelium (Fig. 4c) (Spearman r = 0.44, p < 0.0001). These results are summarised in Supplemental Table 1.
In addition, the HIFs extracted from the cell and tissue models were also used to evaluate features that were distinguished between normal biopsies from those with celiac disease. For example, the proportional area of villous epithelium relative to mucosa and the proportional area of villous epithelium relative to crypt epithelium were both lower in celiac disease tissue compared with normal tissue, while the proportional area of crypt epithelium relative to total epithelium, the proportional area of lamina propria over mucosa, and the density of intraepithelial lymphocytes in villous epithelium were higher in celiac disease (p < 0.0001 for all comparisons) (Table 1).
Discussion
Histological assessment of celiac disease plays a crucial role in diagnosing disease and evaluating the effectiveness of clinical interventions3. However, inter-observer variability can affect the consistency and accuracy of results6. To overcome this limitation and augment pathologists’ assessments of disease severity, we aimed to develop a fully automated and explainable approach to quantify the cellular and tissue-based features of celiac disease in H&E-stained clinical samples. The HIFs extracted from this model reflected histological changes that were measured by modified Marsh scores, potentially providing a quantitative and reproducible means to assess celiac disease severity.
Our model produced continuous feature measurements that can be interpreted as surrogate markers of celiac disease pathology (Supplemental Table 1). The relationship of these features with the ordinal Marsh score categories can be used as a benchmark to measure the model’s performance. For example, we examined the area of villous epithelium relative to the area of mucosa as an indicator of villous blunting, a hallmark of celiac disease, and found a negative correlation with higher modified Marsh scores. To gauge crypt hyperplasia, a more subtle feature, we examined the area of crypt epithelium relative to total epithelial area, revealing a positive correlation between this feature and Marsh scores of 2 and above. The trained cell model directly quantitated the proportion of intraepithelial lymphocytes relative to the number of enterocytes within the villous structures. As expected, these values increased with disease severity. The lower strength of this specific correlation could be attributed to how the modified Marsh score is calculated, in which pathologists assess the presence of > 30 intraepithelial lymphocytes per 100 enterocytes when differentiating scores 0 from 1 rather than quantifying any further increase in intraepithelial lymphocytes as the disease severity increases. In addition, the features extracted from our model align with other clinical trial endpoints such as measurement of villous height and crypt depth4,8. Existing celiac disease scoring systems, such as the modified Marsh score, primarily rely on qualitative and descriptive categorisations, leading to subjectivity and limited sensitivity to subtle changes20. In this study, we propose an alternative approach, utilising ML techniques to enable continuous, quantitative evaluation of the histological changes in celiac disease. By capturing histological alterations on a granular and objective scale, this novel approach offers enhanced sensitivity to changes in intraepithelial lymphocyte density, as well as villous and crypt epithelial surface area, overcoming limitations of the qualitative assessments of conventional manual scoring systems. It is worth noting that there are other scoring systems apart from the Marsh classification for celiac disease, including the Corazza-Villanacci classification7. Future studies to apply digital pathology algorithms to these additional scoring approaches in celiac disease are warranted.
Notably, some of our model-extracted HIFs directly quantified features of intraepithelial lymphocytes. This cell type is an essential consideration during disease assessment, as the presence of > 30 intraepithelial lymphocytes per 100 enterocytes in the duodenum is a defining feature of celiac disease21. The HIFs extracted from our model include count proportions and/or density of intraepithelial lymphocytes, specifically in the villous epithelium. This model also allowed for the extraction of features relating to intraepithelial lymphocytes in crypt epithelium and a comparison of their density in villous and crypt epithelium, providing a comprehensive overview of the spatial distribution of this cell type within distinct epithelial regions. Additional relevant features included the proportional area of villous epithelium (quantifying the change related to villous atrophy), the proportional area of crypt epithelium (quantifying crypt hyperplasia) and the ratio of villous epithelium area to crypt epithelium area (quantitatively capturing the relationship of villous height to crypt depth)22. A decreased epithelial surface area is a long-recognised feature of celiac disease23. Altered crypt surface area represents a histological change that results in symptoms of celiac disease, e.g., malabsorption. While this metric cannot be directly measured by manual pathology approaches, it has been proposed that alternative measurements, such as villous height and crypt length, have the potential to act as surrogate features allowing for villous surface area to be estimated24. Indeed, decreased villous area has previously been associated with increased Marsh score25. The villous and crypt features extracted by our model have the potential to further inform a clinical estimation of small intestine epithelial surface area in celiac disease. This promising methodology should be further investigated as a quantitative approach to measuring the histological features of celiac disease, augmenting traditional approaches to biopsy assessment in this setting.
The key strengths of this study become apparent when considering that these model-generated features not only bear relevance to the modified Marsh scoring system, but are also essential components of the histological hallmarks of celiac disease (Table 1)22. These HIFs encompass features not previously incorporated into any formalised scoring system, such as relative numbers and density of inflammatory cells (including lymphocytes, plasma cells, eosinophils and neutrophils) in lamina propria or in mucosa. These metrics characterise the immune microenvironment within celiac biopsies, as well as the total area and area proportion of lamina propria, capturing the expansion of lamina propria, a phenomenon known to be associated with disease activity22. Furthermore, one of the key strengths of this study lies in our model’s capacity to discern between normal duodenum and celiac disease through the quantification of features associated with the disease microenvironment in mucosal biopsies. As expected, quantifying features of villous atrophy, as evidenced by reduced area proportion of this feature, and the augmented area proportion of crypt epithelium signifying mucosa crypt hyperplasia, distinguished between the histology of unaffected biopsies and those indicative of celiac disease. Supplementary quantitative attributes of the inflammatory microenvironment known to be associated with celiac disease, encompassing the infiltration of chronic inflammatory cells like lymphocytes and plasma cells within the lamina propria, coupled with the associated expansion of this layer22, further distinguished normal biopsy samples from those with celiac disease. Discernible differences between the two groups were also observed in the quantitative evaluation of granulocytes, which has been previously described26,27. Finally, a key, novel aspect of this study is our model’s ability to predict cell types from an H&E-stained WSI. Prior studies have facilitated intraepithelial lymphocyte and lamina propria B cells detection and quantification in celiac disease tissue28,29. However, these studies relied on immunohistochemical staining to detect the cells of interest. The capability to quantify celiac disease features on H&E-stained images, which are routinely used for biopsy assessment, has the potential to streamline the evaluation of clinical celiac disease specimens. While our model was limited by the small sample size, additional assessment involving larger cohorts will allow future refinement of the model’s performance. The cell model can also be trained specifically on duodenum biopsies and expanded to predict features associated with additional cell types, e.g., Paneth cells. An additional limitation of the current approach is related to the extraction of HIFs across a specific tissue area in the entire slide, which overlooks the potential variation in histological disease changes between different tissue fragments, as well as orientation variability. In a manual assessment of celiac disease in biopsies, pathologists often determine disease severity based on the most severely affected tissue region. To address this limitation, future work will focus on reporting HIFs separately for specific regions of interest within the tissue sample. This strategy is expected to allow for a more comprehensive and accurate assessment of disease severity within distinct tissue regions. Finally, our study did not account for orientation variability. The effect of tissue orientation on model-generated HIFs in celiac disease WSIs necessitates further study.
In conclusion, we foresee that ML-supported histological analysis will play a pivotal role in the advancement of precision medicine for patients with celiac disease. To our knowledge, this is the first report of fully explainable ML-based tissue and cell classifications across the WSIs of mucosal biopsies in celiac disease, enabling the extraction and statistical analysis of HIFs to empower translational research and clinical trials. The resulting quantitative model-generated HIFs can be used to build predictive models of existing Marsh scores or function as a continuous measurement, tracking histological change in celiac biopsies. Expanding upon this foundation, as we proceed to develop classification models aimed at predicting clinical outcomes alongside slide-level scores, we anticipate that the interpretability enabled by the utilisation of HIFs is poised to serve a dual purpose: validating the integrity of these models and revealing novel insights into disease biology. We believe that this ML-based assessment has potential as a scalable tool for measuring disease severity, risk stratification, prognostic evaluation, evaluating endpoints in clinical trials, and monitoring of treatment responses—ultimately advancing the care of patients with celiac disease.
Data availability
Model parameters for cell and tissue models, and codes for model training, inference and feature extractions are not disclosed. Access requests for such code will not be considered to safeguard PathAI’s intellectual property. All feature tables, as well as source code, for reproducing correlational analyses will be deposited to GitHub prior to publication, and the link will be provided at that time. Access to cell- and tissue-type heatmaps, as well as usage of cell- and tissue-type classification models, are available on reasonable request to academic investigators, without relevant conflicts of interest, for non-commercial use who agree not to distribute the data. Access requests can be made to: publications@pathai.com.
References
Lebwohl, B., Sanders, D. S. & Green, P. H. R. Coeliac disease. Lancet 391, 70–81 (2018).
Marafini, I., Monteleone, G. & Stolfi, C. Association between celiac disease and cancer. Int. J. Mol. Sci. 21, 4155 (2020).
Rubio-Tapia, A. et al. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 108, 656–76 (2013).
Gottlieb, K., Dawson, J., Hussain, F. & Murray, J. A. Development of drugs for celiac disease: Review of endpoints for Phase 2 and 3 trials. Gastroenterol. Rep. 3, 91–102 (2015).
Green, P. H. R. & Cellier, C. Celiac disease. N. Engl. J. Med. 357, 1731–1743 (2007).
Corazza, G. R. et al. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin. Gastroenterol. Hepatol. 5, 838–843 (2007).
Corazza, G. R. & Villanacci, V. Coeliac disease. J. Clin. Pathol. 58, 573–574 (2005).
Schuppan, D. et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 385, 35–45 (2021).
Lähdeaho, M.-L. et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol 4, 948–959 (2019).
Center for Drug Evaluation & Research. Celiac disease: Developing Drugs for Adjunctive Treatment to a Gluten-free diet. U.S. Food and Drug Administration https://www.fda.gov/regulatory-information/search-fda-guidance-documents/celiac-disease-developing-drugs-adjunctive-treatment-gluten-free-diet (2022).
Ludvigsson, J. F. et al. Outcome measures in coeliac disease trials: The tampere recommendations. Gut 67, 1410–1424 (2018).
Mubarak, A., Nikkels, P., Houwen, R. & Ten Kate, F. Reproducibility of the histological diagnosis of celiac disease. Scand. J. Gastroenterol. 46, 1065–1073 (2011).
Syed, S. et al. Artificial intelligence-based analytics for diagnosis of small bowel enteropathies and black box feature detection. J. Pediatr. Gastroenterol. Nutr. 72, 833–841 (2021).
Rakha, E. A. et al. Current and future applications of artificial intelligence in pathology: A clinical perspective. J. Clin. Pathol. 74, 409–414 (2021).
Harrison, J. H. et al. Introduction to artificial intelligence and machine learning for pathology. Arch. Pathol. Lab. Med. 145, 1228–1254 (2021).
Wei, J. W. et al. Automated detection of celiac disease on duodenal biopsy slides: A deep learning approach. J. Pathol. Inform. 10, 7 (2019).
Koh, J. E. W. et al. Automated interpretation of biopsy images for the detection of celiac disease using a machine learning approach. Comput. Methods Programs Biomed. 203, 106010 (2021).
Najdawi, F. et al. Artificial intelligence enables quantitative assessment of ulcerative colitis histology. Mod. Pathol. 36, 100124 (2023).
Furlanello, C. et al. The development of artificial intelligence in the histological diagnosis of inflammatory bowel disease (IBD-AI). Dig. Liver Dis. https://doi.org/10.1016/j.dld.2024.05.033 (2024).
Adelman, D. C. et al. Measuring change in small intestinal histology in patients with celiac disease. Am. J. Gastroenterol. 113, 339–347 (2018).
Bao, F., Green, P. H. R. & Bhagat, G. An update on celiac disease histopathology and the road ahead. Arch. Pathol. Lab. Med. 136, 735–745 (2012).
Dickson, B. C., Streutker, C. J. & Chetty, R. Coeliac disease: An update for pathologists. J. Clin. Pathol. 59, 1008–1016 (2006).
Guix, M., Skinner, J. M. & Whitehead, R. Measuring intraepithelial lymphocytes, surface area, and volume of lamina propria in the jejunal mucosa of coeliac patients. Gut 20, 275–278 (1979).
Hasan, M. & Ferguson, A. Measurements of intestinal villi non-specific and ulcer-associated duodenitis-correlation between area of microdissected villus and villus epithelial cell count. J. Clin. Pathol. 34, 1181–1186 (1981).
Cummins, A. G. et al. Morphometric evaluation of duodenal biopsies in celiac disease. Am. J. Gastroenterol. 106, 145–150 (2011).
Moran, C. J., Kolman, O. K., Russell, G. J., Brown, I. S. & Mino-Kenudson, M. Neutrophilic infiltration in gluten-sensitive enteropathy is neither uncommon nor insignificant: Assessment of duodenal biopsies from 267 pediatric and adult patients. Am. J. Surg. Pathol. 36, 1339–1345 (2012).
Brown, I. S., Smith, J. & Rosty, C. Gastrointestinal pathology in celiac disease: A case series of 150 consecutive newly diagnosed patients. Am. J. Clin. Pathol. 138, 42–49 (2012).
Taavela, J. et al. Histological, immunohistochemical and mRNA gene expression responses in coeliac disease patients challenged with gluten using PAXgene fixed paraffin-embedded duodenal biopsies. BMC Gastroenterol. 19, 189 (2019).
Popp, A. et al. A new intraepithelial γδ T-lymphocyte marker for celiac disease classification in formalin-fixed paraffin-embedded (FFPE) duodenal biopsies. Dig. Dis. Sci. 66, 3352–3358 (2021).
Acknowledgements
The authors would like to thank all study participants. The authors are grateful to the software engineering and machine learning teams at PathAI for developing the systems and pipelines used for model development and feature extraction and the members of PathAI Pathology Network who contributed manual annotations and scoring to support this work. This study was sponsored by Eli Lilly and Company. Medical writing assistance was provided by Jason Vuong, BPharm, and Clare Weston, MSc, of ProScribe—Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice. Eli Lilly and Company was involved in the study design, oversight and preparation of the manuscript.
Funding
This study was sponsored by Eli Lilly and Company. Medical writing assistance was provided by Jason Vuong, BPharm, and Clare Weston, MSc, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice.
Author information
Authors and Affiliations
Contributions
MG: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, supervision, validation and visualisation; AMG: funding acquisition and writing (review and editing); CS: conceptualisation, data curation, formal analysis, investigation, methodology, validation and visualisation; QW: conceptualisation, data curation, formal analysis, investigation, methodology, validation and visualisation; DF: data curation; AK: conceptualisation, investigation, methodology, software and writing (review and editing); CK: conceptualisation, data curation and writing (review and editing); DB: conceptualisation, investigation, formal analysis and writing (review and editing); JABC: writing (original draft) and writing (review and editing); CJ: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, validation and visualisation; FN: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, validation, visualisation, writing (original draft) and writing (review and editing); KG: conceptualisation, funding acquisition, supervision and writing (review and editing). All authors: final approval of the manuscript. FN and KG are joint senior/last authors.
Corresponding author
Ethics declarations
Competing interest
AMG, ADF, KMC, and KG are employees and shareholders of Eli Lilly and Company. MG, CS, QS, DF, AK, CK, DB, JABC, CJ, and FN are shareholders in and current or former employees of PathAI. QW and FN were employees of PathAI at the time of study.
Ethical approval
This study was conducted as specified in WCG IRB protocol number 1316112.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Griffin, M., Gruver, A.M., Shah, C. et al. A feasibility study using quantitative and interpretable histological analyses of celiac disease for automated cell type and tissue area classification. Sci Rep 14, 29883 (2024). https://doi.org/10.1038/s41598-024-79570-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79570-1