A novel vascular smooth muscle chymase is upregulated in hypertensive rats
- PMID: 11254670
- PMCID: PMC208939
- DOI: 10.1172/JCI9997
A novel vascular smooth muscle chymase is upregulated in hypertensive rats
Abstract
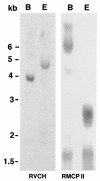
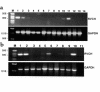
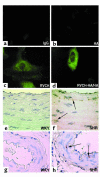
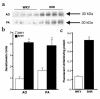
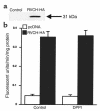
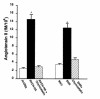
While greater than 80% of angiotensin II (Ang II) formation in the human heart and greater than 60% in arteries appears to result from chymase activity, no cardiovascular cell-expressed chymase has been previously reported. We now describe the cloning of a full-length cDNA encoding a novel chymase from rat vascular smooth muscle cells. The cDNA encompasses 953 nucleotides, encodes 247 amino acids, and exhibits 74% and 80% homology in amino acid sequence to rat mast cell chymase I and II, respectively. Southern blot analysis indicates that the rat vascular chymase is encoded by a separate gene. This chymase was induced in hypertrophied rat pulmonary arteries, with 11-fold and 8-fold higher chymase mRNA levels in aortic and pulmonary artery smooth muscle cells from spontaneously hypertensive than in corresponding tissues from normotensive rats. We assayed the activity of the endogenous enzyme and of a recombinant, epitope-tagged chymase in transfected smooth muscle cells and showed that Ang II production from Ang I can be inhibited with chymostatin, but not EDTA or captopril. Spontaneously hypertensive rats show elevated chymase expression and increased chymostatin-inhibitable angiotensin-converting activity, suggesting a possible role for this novel enzyme in the pathophysiology of hypertension.
Figures






Similar articles
-
Role of chymase-dependent angiotensin II formation in regulating blood pressure in spontaneously hypertensive rats.Hypertens Res. 2005 May;28(5):457-64. doi: 10.1291/hypres.28.457. Hypertens Res. 2005. PMID: 16156510
-
Conditional and _targeted overexpression of vascular chymase causes hypertension in transgenic mice.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7469-74. doi: 10.1073/pnas.131147598. Proc Natl Acad Sci U S A. 2001. PMID: 11416217 Free PMC article.
-
Angiotensin converting enzyme-independent angiotensin ii production by chymase is up-regulated in the ischemic kidney in renovascular hypertension.J Surg Res. 2005 Aug;127(2):65-9. doi: 10.1016/j.jss.2005.02.031. J Surg Res. 2005. PMID: 15869764
-
Vascular chymase: pathophysiological role and therapeutic potential of inhibition.Cardiovasc Res. 2004 Mar 1;61(4):653-62. doi: 10.1016/j.cardiores.2003.11.029. Cardiovasc Res. 2004. PMID: 14985062 Review.
-
[Progress in the study of chymase].Sheng Li Ke Xue Jin Zhan. 1996 Apr;27(2):129-33. Sheng Li Ke Xue Jin Zhan. 1996. PMID: 9592235 Review. Chinese.
Cited by
-
Mast Cell and Basophil Granule Proteases - In Vivo _targets and Function.Front Immunol. 2022 Jul 5;13:918305. doi: 10.3389/fimmu.2022.918305. eCollection 2022. Front Immunol. 2022. PMID: 35865537 Free PMC article. Review.
-
Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin.J Clin Invest. 2002 Jun;109(11):1417-27. doi: 10.1172/JCI14276. J Clin Invest. 2002. PMID: 12045255 Free PMC article.
-
Cellular phenotypes and the genetics of hypertension.Curr Hypertens Rep. 2002 Feb;4(1):32-6. doi: 10.1007/s11906-002-0050-1. Curr Hypertens Rep. 2002. PMID: 11790289 Review.
-
Mutations in Arg143 and Lys192 of the Human Mast Cell Chymase Markedly Affect the Activity of Five Potent Human Chymase Inhibitors.PLoS One. 2013 Jun 19;8(6):e65988. doi: 10.1371/journal.pone.0065988. Print 2013. PLoS One. 2013. PMID: 23840386 Free PMC article.
-
Big ET-1 processing into vasoactive peptides in arteries and veins.Vascul Pharmacol. 2007 Nov-Dec;47(5-6):302-12. doi: 10.1016/j.vph.2007.08.006. Epub 2007 Sep 7. Vascul Pharmacol. 2007. PMID: 17904426 Free PMC article.
References
-
- Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–H1194. - PubMed
-
- Rosendorff C. Vascular hypertrophy in hypertension: role of the renin-angiotensin system. Mt Sinai J Med. 1998;65:108–117. - PubMed
-
- Curzen NP, Fox KM. Do ACE inhibitors modulate atherosclerosis? Eur Heart J. 1997;18:1530–1535. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

