Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals
- PMID: 19019002
- PMCID: PMC6640315
- DOI: 10.1111/j.1364-3703.2008.00500.x
Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals
Abstract
Phytophthora ramorum is an oomycete plant pathogen classified in the kingdom Stramenopila. P. ramorum is the causal agent of sudden oak death on coast live oak and tanoak as well as ramorum blight on woody ornamental and forest understorey plants. It causes stem cankers on trees, and leaf blight or stem dieback on ornamentals and understorey forest species. This pathogen is managed in the USA and Europe by eradication where feasible, by containment elsewhere and by quarantine in many parts of the world. Genomic resources provide information on genes of interest to disease management and have improved tremendously since sequencing the genome in 2004. This review provides a current overview of the pathogenicity, population genetics, evolution and genomics of P. ramorum.
Taxonomy: Phytophthora ramorum (Werres, De Cock & Man in't Veld): kingdom Stramenopila; phylum Oomycota; class Peronosporomycetidae; order Pythiales; family Pythiaceae; genus Phytophthora.
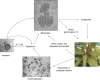
Host range: The host range is very large and the list of known hosts continues to expand at the time of writing. Coast live oak and tanoak are ecologically, economically and culturally important forest hosts in the USA. Rhododendron, Viburnum, Pieris, Syringa and Camellia are key ornamental hosts on which P. ramorum has been found repeatedly, some of which have been involved in moving the pathogen via nursery shipments. Disease symptoms: P. ramorum causes two different diseases with differing symptoms: sudden oak death (bleeding lesions, stem cankers) on oaks and ramorum blight (twig dieback and/or foliar lesions) on tree and woody ornamental hosts.
Useful websites: http://nature.berkeley.edu/comtf/, http://rapra.csl.gov.uk/, http://www.aphis.usda.gov/plant_health/plant_pest_info/pram/index.shtml, http://genome.jgi-psf.org/Phyra1_1/Phyra1_1.home.html, http://pamgo.vbi.vt.edu/, http://pmgn.vbi.vt.edu/, http://vmd.vbi.vt.edu./, http://web.science.oregonstate.edu/bpp/labs/grunwald/resources.htm, http://www.defra.gov.uk/planth/pramorum.htm, http://www.invasive.org/browse/subject.cfm?sub=4603, http://www.forestry.gov.uk/forestry/WCAS-4Z5JLL.
Figures




Similar articles
-
Sudden oak death: interactions of the exotic oomycete Phytophthora ramorum with naïve North American hosts.Eukaryot Cell. 2012 Nov;11(11):1313-23. doi: 10.1128/EC.00195-12. Epub 2012 Sep 21. Eukaryot Cell. 2012. PMID: 23002108 Free PMC article. Review.
-
Phytophthora sojae: root rot pathogen of soybean and model oomycete.Mol Plant Pathol. 2007 Jan;8(1):1-8. doi: 10.1111/j.1364-3703.2006.00373.x. Mol Plant Pathol. 2007. PMID: 20507474
-
Phytophthora ramorum on Quercus ilex in the United Kingdom.Plant Dis. 2005 Nov;89(11):1241. doi: 10.1094/PD-89-1241A. Plant Dis. 2005. PMID: 30786450
-
First report of the NA2 clonal lineage of the sudden oak death pathogen, Phytophthora ramorum, infecting tanoak in Oregon forests.Plant Dis. 2022 Feb 11. doi: 10.1094/PDIS-10-21-2152-PDN. Online ahead of print. Plant Dis. 2022. PMID: 35147452
-
Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests.Annu Rev Phytopathol. 2005;43:309-35. doi: 10.1146/annurev.phyto.42.040803.140418. Annu Rev Phytopathol. 2005. PMID: 16078887 Review.
Cited by
-
Fire, CO2, and climate effects on modeled vegetation and carbon dynamics in western Oregon and Washington.PLoS One. 2019 Jan 25;14(1):e0210989. doi: 10.1371/journal.pone.0210989. eCollection 2019. PLoS One. 2019. PMID: 30682107 Free PMC article.
-
Multiplex LAMP Detection of the Genus Phytophthora and Four Phytophthora Species P. ramorum, P. lateralis, P. kernoviae, and P. nicotianae, with a Plant Internal Control.Microbes Environ. 2021;36(2):ME21019. doi: 10.1264/jsme2.ME21019. Microbes Environ. 2021. PMID: 34108359 Free PMC article.
-
Genera of phytopathogenic fungi: GOPHY 4.Stud Mycol. 2022 Jul;101:417-564. doi: 10.3114/sim.2022.101.06. Epub 2022 Jun 2. Stud Mycol. 2022. PMID: 36059898 Free PMC article.
-
Sudden oak death: interactions of the exotic oomycete Phytophthora ramorum with naïve North American hosts.Eukaryot Cell. 2012 Nov;11(11):1313-23. doi: 10.1128/EC.00195-12. Epub 2012 Sep 21. Eukaryot Cell. 2012. PMID: 23002108 Free PMC article. Review.
-
The Top 10 oomycete pathogens in molecular plant pathology.Mol Plant Pathol. 2015 May;16(4):413-34. doi: 10.1111/mpp.12190. Epub 2014 Dec 11. Mol Plant Pathol. 2015. PMID: 25178392 Free PMC article. Review.
References
-
- Allen, R.L. , Bittner‐Eddy, P.D. , Grenville‐Briggs, L.J. , Meitz, J.C. , Rehmany, A.P. , Rose, L.E. and Beynon, J.L. (2004) Host–parasite coevolutionary conflict between arabidopsis and downy mildew. Science, 306, 1957–1960. - PubMed
-
- Anacker, B.L. , Rank, N.E. , Huberli, D. , Garbelotto, M. , Gordon, S. , Harnik, T. , Whitkus, R. and Meentemeyer, R. (2008) Susceptibility to Phytophthora ramorum in a key infectious host: landscape variation in host genotype, host phenotype, and environmental factors. New Phytol. 177, 756–766. - PubMed
-
- Avila‐Adame, C. , Gomez‐Alpizar, L. , Zismann, V. , Jones, K.M. , Buell, C.R. and Ristaino, J.B. (2006) Mitochondrial genome sequences and molecular evolution of the Irish potato famine pathogen, Phytophthora infestans . Curr. Genet. 49, 39–46. - PubMed
-
- Baldauf, S.L. (2003) The deep roots of eukaryotes. Science, 300, 1703–1706. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

