Gum acacia-based silver nanoparticles as a highly selective and sensitive dual nanosensor for Hg(ii) and fluorescence turn-off sensor for S2- and malachite green detection
- PMID: 35497744
- PMCID: PMC9048504
- DOI: 10.1039/c9ra10372d
Gum acacia-based silver nanoparticles as a highly selective and sensitive dual nanosensor for Hg(ii) and fluorescence turn-off sensor for S2- and malachite green detection
Abstract
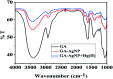
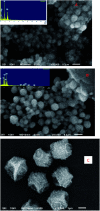
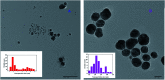
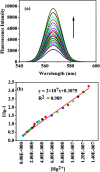
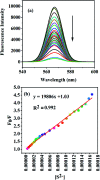
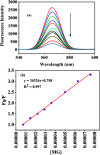
A facile and green method was adopted to synthesize highly selective gum acacia-mediated silver nanoparticles as dual sensor (fluorescence turn-on and colorimetric) for Hg(ii) and fluorescence turn-off sensor for S2- and malachite green. The mechanism proposed for a dual response towards Hg(ii) is the redox reaction between Ag(0) and Hg(ii), resulting in the formation of Ag(i) and Hg(0) and electron transfer from gum acacia to Ag(i), which further leads to the formation of an Ag@Hg nanoalloy. The enhanced fluorescence signal was quenched selectively by S2- owing to the formation of Ag2S and HgS. The reported nanosensor was found to be useful for sensing malachite green via the inner filter effect. The linear ranges were 3 nmol L-1 to 13 μmol L-1 for Hg(ii), 3-170 μmol L-1 for S2- and 7-80 μmol L-1 for malachite green, and the corresponding detection limits were 2.1 nmol L-1 for Hg(ii), 1.3 μmol L-1 for S2- and 1.6 μmol L-1 for malachite green.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Gum kondagogu reduced/stabilized silver nanoparticles as direct colorimetric sensor for the sensitive detection of Hg²⁺ in aqueous system.Talanta. 2014 Jan;118:111-7. doi: 10.1016/j.talanta.2013.10.012. Epub 2013 Oct 11. Talanta. 2014. PMID: 24274277
-
Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag(+) and Hg(2+).Anal Chim Acta. 2015 Apr 22;870:1-7. doi: 10.1016/j.aca.2015.01.016. Epub 2015 Jan 14. Anal Chim Acta. 2015. PMID: 25819783
-
A sensitive turn-off-on fluorometric sensor based on S,N co-doped carbon dots for environmental analysis of Hg(II) ion.Luminescence. 2021 Aug;36(5):1151-1158. doi: 10.1002/bio.4040. Epub 2021 Mar 27. Luminescence. 2021. PMID: 33686780
-
Fluorescence resonance energy transfer between carbon quantum dots and silver nanoparticles: Application to mercuric ion sensing.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jan 15;245:118924. doi: 10.1016/j.saa.2020.118924. Epub 2020 Sep 9. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 32950856
-
A highly efficient and selective turn-on fluorescent sensor for Hg2+, Ag+ and Ag nanoparticles based on a coumarin dithioate derivative.Luminescence. 2014 Mar;29(2):158-67. doi: 10.1002/bio.2521. Epub 2013 May 22. Luminescence. 2014. PMID: 23703858
Cited by
-
o-phenylenediamine Derived Fluorescent Carbon Quantum dots for Detection of Hg(II) in Environmental Water.J Fluoresc. 2024 Mar;34(2):905-913. doi: 10.1007/s10895-023-03331-y. Epub 2023 Jul 7. J Fluoresc. 2024. PMID: 37418199
-
Enhanced Sensitivity and Accuracy of Tb3+-Functionalized Zirconium-Based Bimetallic MOF for Visual Detection of Malachite Green in Fish.Foods. 2024 Sep 9;13(17):2855. doi: 10.3390/foods13172855. Foods. 2024. PMID: 39272620 Free PMC article.
-
Captivating nano sensors for mercury detection: a promising approach for monitoring of toxic mercury in environmental samples.RSC Adv. 2024 Jun 12;14(27):18907-18941. doi: 10.1039/d4ra02787f. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38873550 Free PMC article. Review.
References
-
- Annadhasan M. Rajendiran N. RSC Adv. 2015;5:94513–94518. doi: 10.1039/C5RA18106B. - DOI
-
- Bell L. DiGangi J. Weinberg J. Ipen. 2014:1–209.
LinkOut - more resources
Full Text Sources

