Dietary glucosamine overcomes the defects in αβ-T cell ontogeny caused by the loss of de novo hexosamine biosynthesis
- PMID: 36456551
- PMCID: PMC9715696
- DOI: 10.1038/s41467-022-35014-w
Dietary glucosamine overcomes the defects in αβ-T cell ontogeny caused by the loss of de novo hexosamine biosynthesis
Abstract
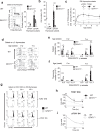
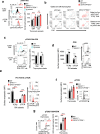
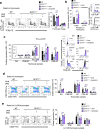
T cell development requires the coordinated rearrangement of T cell receptor (TCR) gene segments and the expression of either αβ or γδ TCR. However, whether and how de novo synthesis of nutrients contributes to thymocyte commitment to either lineage remains unclear. Here, we find that T cell-specific deficiency in glutamine:fructose-6-phosphate aminotransferase 1 (GFAT1), the rate-limiting enzyme of the de novo hexosamine biosynthesis pathway (dn-HBP), attenuates hexosamine levels, blunts N-glycosylation of TCRβ chains, reduces surface expression of key developmental receptors, thus impairing αβ-T cell ontogeny. GFAT1 deficiency triggers defects in N-glycans, increases the unfolded protein response, and elevates γδ-T cell numbers despite reducing γδ-TCR diversity. Enhancing TCR expression or PI3K/Akt signaling does not reverse developmental defects. Instead, dietary supplementation with the salvage metabolite, glucosamine, and an α-ketoglutarate analogue partially restores αβ-T cell development in GFAT1T-/- mice, while fully rescuing it in ex vivo fetal thymic organ cultures. Thus, dn-HBP fulfils, while salvage nutrients partially satisfy, the elevated demand for hexosamines during early T cell development.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The Hexosamine Biosynthesis Pathway: Regulation and Function.Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review.
-
Human alpha beta and gamma delta thymocyte development: TCR gene rearrangements, intracellular TCR beta expression, and gamma delta developmental potential--differences between men and mice.J Immunol. 2006 Feb 1;176(3):1543-52. doi: 10.4049/jimmunol.176.3.1543. J Immunol. 2006. PMID: 16424183 Free PMC article.
-
Evidence that gammadelta versus alphabeta T cell fate determination is initiated independently of T cell receptor signaling.J Exp Med. 2001 Mar 19;193(6):689-98. doi: 10.1084/jem.193.6.689. J Exp Med. 2001. PMID: 11257136 Free PMC article.
-
Deep sequencing of the human TCRγ and TCRβ repertoires suggests that TCRβ rearranges after αβ and γδ T cell commitment.Sci Transl Med. 2011 Jul 6;3(90):90ra61. doi: 10.1126/scitranslmed.3002536. Sci Transl Med. 2011. PMID: 21734177 Free PMC article.
-
αβ versus γδ fate choice: counting the T-cell lineages at the branch point.Immunol Rev. 2010 Nov;238(1):169-81. doi: 10.1111/j.1600-065X.2010.00947.x. Immunol Rev. 2010. PMID: 20969592 Free PMC article. Review.
Cited by
-
Glycome dynamics in T and B cell development: basic immunological mechanisms and clinical applications.Trends Immunol. 2023 Aug;44(8):585-597. doi: 10.1016/j.it.2023.06.004. Epub 2023 Jul 4. Trends Immunol. 2023. PMID: 37407365 Free PMC article. Review.
-
Novel Approaches in Chronic Renal Failure without Renal Replacement Therapy: A Review.Biomedicines. 2023 Oct 18;11(10):2828. doi: 10.3390/biomedicines11102828. Biomedicines. 2023. PMID: 37893201 Free PMC article. Review.
-
The mTORC2 signaling network: _targets and cross-talks.Biochem J. 2024 Jan 25;481(2):45-91. doi: 10.1042/BCJ20220325. Biochem J. 2024. PMID: 38270460 Free PMC article. Review.
-
The Hexosamine Biosynthesis Pathway: Regulation and Function.Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review.
References
-
- Fahl, S. P., Kappes, D. J. & Wiest, D. L. In Signaling Mechanisms Regulating T Cell Diversity and Function (eds J. Soboloff & D. J. Kappes) 85–104 (CRC Press/Taylor & Francis, 2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

