Aging and aging-related diseases: from molecular mechanisms to interventions and treatments
- PMID: 36522308
- PMCID: PMC9755275
- DOI: 10.1038/s41392-022-01251-0
Aging and aging-related diseases: from molecular mechanisms to interventions and treatments
Abstract
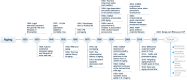
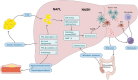
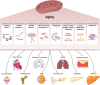
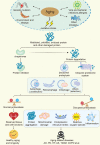
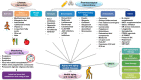
Aging is a gradual and irreversible pathophysiological process. It presents with declines in tissue and cell functions and significant increases in the risks of various aging-related diseases, including neurodegenerative diseases, cardiovascular diseases, metabolic diseases, musculoskeletal diseases, and immune system diseases. Although the development of modern medicine has promoted human health and greatly extended life expectancy, with the aging of society, a variety of chronic diseases have gradually become the most important causes of disability and death in elderly individuals. Current research on aging focuses on elucidating how various endogenous and exogenous stresses (such as genomic instability, telomere dysfunction, epigenetic alterations, loss of proteostasis, compromise of autophagy, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, deregulated nutrient sensing) participate in the regulation of aging. Furthermore, thorough research on the pathogenesis of aging to identify interventions that promote health and longevity (such as caloric restriction, microbiota transplantation, and nutritional intervention) and clinical treatment methods for aging-related diseases (depletion of senescent cells, stem cell therapy, antioxidative and anti-inflammatory treatments, and hormone replacement therapy) could decrease the incidence and development of aging-related diseases and in turn promote healthy aging and longevity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Ageing as a risk factor for neurodegenerative disease.Nat Rev Neurol. 2019 Oct;15(10):565-581. doi: 10.1038/s41582-019-0244-7. Epub 2019 Sep 9. Nat Rev Neurol. 2019. PMID: 31501588 Review.
-
The hallmarks of aging.Cell. 2013 Jun 6;153(6):1194-217. doi: 10.1016/j.cell.2013.05.039. Cell. 2013. PMID: 23746838 Free PMC article. Review.
-
Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms.Theranostics. 2022 Mar 6;12(6):2722-2740. doi: 10.7150/thno.71360. eCollection 2022. Theranostics. 2022. PMID: 35401820 Free PMC article. Review.
-
Biomolecular Markers of Brain Aging.Adv Exp Med Biol. 2023;1419:111-126. doi: 10.1007/978-981-99-1627-6_9. Adv Exp Med Biol. 2023. PMID: 37418210
-
Mechanistic links between aging and lung fibrosis.Biogerontology. 2013 Dec;14(6):609-15. doi: 10.1007/s10522-013-9451-6. Epub 2013 Aug 9. Biogerontology. 2013. PMID: 23929205 Free PMC article. Review.
Cited by
-
HIF-1α knockdown attenuates phenotypic transformation and oxidative stress induced by high salt in human aortic vascular smooth muscle cells.Sci Rep. 2024 Nov 15;14(1):28100. doi: 10.1038/s41598-024-79892-0. Sci Rep. 2024. PMID: 39543255 Free PMC article.
-
Physical and mental well-being of older adults: examining the impact of financial support from male migrant children on Indian left-behind parents.Arch Public Health. 2024 Nov 14;82(1):214. doi: 10.1186/s13690-024-01413-2. Arch Public Health. 2024. PMID: 39538319 Free PMC article.
-
Body Composition and Senescence: Impact of Polyphenols on Aging-Associated Events.Nutrients. 2024 Oct 25;16(21):3621. doi: 10.3390/nu16213621. Nutrients. 2024. PMID: 39519454 Free PMC article. Review.
-
Recent Insights into Endogenous Mammalian Cardiac Regeneration Post-Myocardial Infarction.Int J Mol Sci. 2024 Nov 1;25(21):11747. doi: 10.3390/ijms252111747. Int J Mol Sci. 2024. PMID: 39519298 Free PMC article. Review.
-
Proximity Elongation Assay and ELISA for the Identification of Serum Diagnostic Biomarkers in Parkinson's Disease and Progressive Supranuclear Palsy.Int J Mol Sci. 2024 Oct 30;25(21):11663. doi: 10.3390/ijms252111663. Int J Mol Sci. 2024. PMID: 39519214 Free PMC article.
References
-
- McCay CM, Maynard LA, Sperling G, Barnes LL. The Journal of Nutrition. Volume 18 July-December, 1939. Pages 1–13. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. Nutr. Rev. 1975;33:241–243. doi: 10.1111/j.1753-4887.1975.tb05227.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

