Furanocoumarin Notopterol: Inhibition of Hepatocellular Carcinogenesis through Suppression of Cancer Stemness Signaling and Induction of Oxidative Stress-Associated Cell Death
- PMID: 37299411
- PMCID: PMC10255513
- DOI: 10.3390/nu15112447
Furanocoumarin Notopterol: Inhibition of Hepatocellular Carcinogenesis through Suppression of Cancer Stemness Signaling and Induction of Oxidative Stress-Associated Cell Death
Abstract
Background: Hepatocellular carcinoma (HCC) remains an aggressive malignancy with a poor prognosis and a leading cause of cancer-related mortality globally. Cumulative evidence suggests critical roles for endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in chronic liver diseases. However, the role of ER stress in HCC pathogenesis, aggressiveness and therapy response remains unclear and understudied.
Objectives: Against this background, the present study evaluated the therapeutic efficacy and feasibility of notopterol (NOT), a furanocoumarin and principal component of Notopterygium incisum, in the modulation of ER stress and cancer stemness, and the subsequent effect on liver oncogenicity.
Methods: An array of biomolecular methods including Western blot, drug cytotoxicity, cell motility, immunofluorescence, colony and tumorsphere formation, flow-cytometric mitochondrial function, GSH/GSSG ratio, and tumor xenograft ex vivo assays were used in the study.
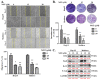
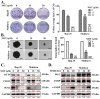
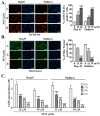
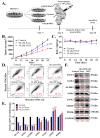
Results: Herein, we demonstrated that NOT significantly suppresses the viability, migration, and invasion capacity of the human HCC HepJ5 and Mahlavu cell lines by disrupting ATF4 expression, inhibiting JAK2 activation, and downregulating the GPX1 and SOD1 expression in vitro. NOT also markedly suppressed the expression of vimentin (VIM), snail, b-catenin, and N-cadherin in the HCC cells, dose-dependently. Treatment with NOT significantly attenuated cancer stem cells (CSCs)-like phenotypes, namely colony and tumorsphere formation, with the concomitant downregulation of stemness markers OCT4, SOX2, CD133, and upregulated PARP-1 cleavage, dose-dependently. We also demonstrated that NOT anticancer activity was strongly associated with increased cellular reactive oxidative stress (ROS) but, conversely, reduced mitochondrial membrane potential and function in the HepJ5 and Mahlavu cells in vitro. Our tumor xenograft studies showed that compared with sorafenib, NOT elicited greater tumor growth suppression without adverse changes in mice body weights. Compared with the untreated control and sorafenib-treated mice, NOT-treated mice exhibited markedly greater apoptosis ex vivo, and this was associated with the co-suppression of stemness and drug-resistance markers OCT4, SOX2, ALDH1, and the upregulation of endoplasmic reticulum stress and oxidative stress factors PERK and CHOP.
Conclusions: In summary, we demonstrated for the first time that NOT exhibits strong anticancer activity via the suppression of cancer stemness, enhanced endoplasmic reticulum stress and increased oxidative stress thus projecting NOT as a potentially effective therapeutic agent against HCC.
Keywords: HCC; anticancer treatment; cancer stemness; endoplasmic reticulum stress; hepatocellular carcinoma; notopterol; oxidative stress.
Conflict of interest statement
The authors declare that they have no potential financial competing interests that may in any way, gain or lose financially from the publication of this manuscript at present or in the future. Additionally, no non-financial competing interests are involved in the manuscript.
Figures


Similar articles
-
Melatonin Increases the Sensitivity of Hepatocellular Carcinoma to Sorafenib through the PERK-ATF4-Beclin1 Pathway.Int J Biol Sci. 2019 Jul 21;15(9):1905-1920. doi: 10.7150/ijbs.32550. eCollection 2019. Int J Biol Sci. 2019. PMID: 31523192 Free PMC article.
-
Elevated PDK1 Expression Drives PI3K/AKT/MTOR Signaling Promotes Radiation-Resistant and Dedifferentiated Phenotype of Hepatocellular Carcinoma.Cells. 2020 Mar 18;9(3):746. doi: 10.3390/cells9030746. Cells. 2020. PMID: 32197467 Free PMC article.
-
Sorafenib enhances proteasome inhibitor-mediated cytotoxicity via inhibition of unfolded protein response and keratin phosphorylation.Exp Cell Res. 2013 Aug 15;319(14):2166-78. doi: 10.1016/j.yexcr.2013.05.023. Epub 2013 May 31. Exp Cell Res. 2013. PMID: 23727131
-
Endoplasmic Reticulum Stress Signaling and the Pathogenesis of Hepatocarcinoma.Int J Mol Sci. 2021 Feb 11;22(4):1799. doi: 10.3390/ijms22041799. Int J Mol Sci. 2021. PMID: 33670323 Free PMC article. Review.
-
Endoplasmic reticulum stress: Multiple regulatory roles in hepatocellular carcinoma.Biomed Pharmacother. 2021 Oct;142:112005. doi: 10.1016/j.biopha.2021.112005. Epub 2021 Aug 20. Biomed Pharmacother. 2021. PMID: 34426262 Review.
Cited by
-
Phytochemistry and Biological Profile of the Chinese Endemic Herb Genus Notopterygium.Molecules. 2024 Jul 9;29(14):3252. doi: 10.3390/molecules29143252. Molecules. 2024. PMID: 39064831 Free PMC article. Review.
-
The dual effect of endoplasmic reticulum stress in digestive system tumors and intervention of Chinese botanical drug extracts: a review.Front Pharmacol. 2024 Feb 21;15:1339146. doi: 10.3389/fphar.2024.1339146. eCollection 2024. Front Pharmacol. 2024. PMID: 38449811 Free PMC article. Review.
-
A review on the mechanisms underlying the antitumor effects of natural products by _targeting the endoplasmic reticulum stress apoptosis pathway.Front Pharmacol. 2023 Nov 17;14:1293130. doi: 10.3389/fphar.2023.1293130. eCollection 2023. Front Pharmacol. 2023. PMID: 38044941 Free PMC article. Review.
-
Notopterol Suppresses IL-17-Induced Proliferation and Invasion of A549 Lung Adenocarcinoma Cells via Modulation of STAT3, NF-κB, and AP-1 Activation.Int J Mol Sci. 2023 Oct 11;24(20):15057. doi: 10.3390/ijms242015057. Int J Mol Sci. 2023. PMID: 37894738 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

